Patients who undergo treatment for head and neck cancers often suffer from acute or late reactions to therapy. Severity of these oral complications may be based on the location and extent of tumor, as well as the type and extent of treatment. Some complications are transient, whereas others require a lifetime of management secondary to damage that results in permanent dysfunction. Patients who have a history of head and neck cancers are also at an increased risk for recurrences or second malignancies, and therefore require close follow-up. Dental professionals should provide preventive and supportive care, including education and symptom management, for patients experiencing oral complications related to cancer therapy, and should closely monitor patients’ level of distress, ability to cope, and treatment response.
Structures in the head and neck are often negatively impacted by surgery, cancer chemotherapy, and ionizing radiation, common therapies for head and neck cancer . Oral complications may develop from both direct damage to oral tissues as a result of radiation therapy (RT) and chemotherapy (CT), and indirect damage caused by regional or systemic toxicity that secondarily affects the oral cavity and surrounding structures . Therapy-related toxicity to oral structures can be exacerbated by colonizing oral microflora when local and systemic immune function is concurrently compromised .
Oral complications occur during and following courses of RT, CT, and combined chemoradiotherapy treatment for head and neck cancer ( Table 1 ). Acute reactions occur during the course of therapy, primarily caused by direct toxicity, and resolve over weeks to months following the completion of CT or RT. Chronic or late reactions occur months and years after RT or CT has ended. RT in particular induces permanent tissue damage that results in lifelong morbidity for the patient. Such complications are the result of a change in vascular supply, fibrosis in connective tissue and muscle, neuropathy, mucosal atrophy, and change in the cellularity of tissues . Hyperfractionation of radiation therapy may reduce the late complications, but increases the severity of the acute reactions.
| Oral complication | Risk factor |
|---|---|
| Acute reactions | |
| Surgery-related pain and defects | Surgical procedure |
| Oral mucositis | Mucosal cytotoxicity |
| Physical/chemical trauma | |
| Decreased local/systemic immunity | |
| Oral Infections | |
| Fungal | Decreased systemic immunity |
| Salivary gland dysfunction | |
| Altered oral flora | |
| Bacterial | Decreased systemic immunity |
| Salivary gland dysfunction | |
| Inadequate oral hygiene | |
| Mucosal breakdown | |
| Acquired pathogens | |
| Viral | Decreased systemic immunity |
| Hyposalivation | Salivary gland toxicity |
| Neurotoxicity | Vinca alkaloid drug use; specific drug toxicity |
| Late reactions | |
| Oral infections | |
| Fungal | Salivary gland dysfunction |
| Altered oral flora | |
| Salivary gland dysfunction | |
| Bacterial | Inadequate oral hygiene |
| Mucosal breakdown | |
| Acquired pathogens | |
| Salivary gland toxicity | |
| Hyposalivation | Salivary gland toxicity |
| Chronic mucosal changes | Mucosal toxicity |
| Submucosal fibrosis | |
| Caries | Hyposalivation |
| Altered oral flora | |
| Soft-tissue and post-radiation osteonecrosis | Mucosal and bone toxicity |
| Trismus and muscle pain | Fibrosis |
| Surgical changes | |
| Speech and mastication problems | Surgical changes |
| Fibrosis | |
| Hyposalivation | |
| Taste dysfunction | Taste receptor toxicity |
| Chronic pain | Surgery, radiation, chemotherapy |
| Dentofacial abnormalities | Radiation, chemotherapy |
Acute reactions
Surgical procedures
Acute pain is expected secondary to surgical procedures for head and neck cancer. Surgery-related pain usually involves acute inflammatory responses related to the extent of the surgery, and may be associated with a variable degree of concomitant nerve injury. As with any surgical intervention, primary closure allows for optimal wound healing. Advances in surgical management include new approaches to reconstruction, such as vascularized flaps, free microvascular reconstruction, and neurologic anastomoses of free grafts .
Oral mucositis
Oral mucositis is a common acute complication resulting from CT or RT/CT, and typically manifests as erythema or ulceration of the oral mucosa. It is a painful and debilitating condition that is a dose- and rate-limiting toxicity of cancer therapy. Other potential sequelae of mucositis include increased risk for local and systemic infection and compromised oral and pharyngeal function, thereby affecting the patient’s total quality of life . The trend toward more aggressive therapy to improve cancer cure rates has increased the frequency, severity, and duration of oral mucositis (see the article by Lalla and colleagues elsewhere in this issue).
In RT, oral mucositis is the result of cumulative tissue dose, and is almost universal in patients undergoing treatment involving the oropharynx. This acute reaction occurs secondary to radiation-induced mitotic death of the basal cells in the oral mucosa, when destruction of these epithelial cells surpasses the rate of proliferation . Unlike CT, radiation damage is anatomically site-specific, and toxicity is localized to irradiated tissue ( Fig. 1 ). The degree of damage depends on treatment regimen-related factors, including type of radiation used, total dose administered, field site, and field size/fractionation. Pain often escalates at week 3, peaking at week 5, and persisting for weeks, with gradual remission of signs and symptoms . Duration of radiation-induced oral mucositis typically extends for 6 to 8 weeks . Radiation-induced damage also differs from chemotherapy-induced changes in that irradiated tissue tends to manifest permanent damage that places the patient at continual and lifelong risk for oral sequelae. The oral tissues are thus more easily damaged by subsequent toxic drug or radiation exposure, a condition known as radiation recall injury, and normal physiologic repair mechanisms are compromised as a result of permanent cellular damage . A retrospective cohort study of 204 patients who underwent RT found that oral mucositis was associated with increased weight loss and an incremental increase in treatment costs, depending upon the severity of mucositis . Chemoradiotherapy has been documented to result in increased frequency, severity, and duration of mucositis .
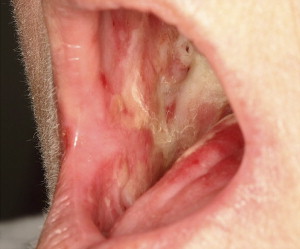
Hyposalivation
Chemotherapeutic agents may cause transient damage to salivary glands. It is unclear which chemotherapeutic agents cause hyposalivation, but approximately 40% of patients will report this side effect during therapy . It is usually short term, and complete recovery is noted 2 to 8 weeks after therapy.
Ionizing radiation can cause irreparable damage to salivary glands that are included in the radiation fields. Whereas reversible effects may occur at doses of 20 to 30 Gy, irreversible effects occur at total doses of greater than 50 Gy, resulting in inflammatory and degenerative changes of salivary acinar cells, alteration in duct epithelium, and fibrosis of the connective tissue . During RT, the serous acini are affected earlier than the mucinous acini, resulting in a thick viscous salivary secretion.
Radiation-induced hyposalivation starts early during treatment. In the first week, a 50% to 60% decrease in salivary flow may occur, and after 7 weeks of conventional RT, salivary flow diminishes to approximately 20% . Following RT, salivary gland recovery is possible until 12 to 18 months following treatment, depending on the dose and volume of gland tissue included in the irradiation fields. Regardless, recovery is usually incomplete, and reduced salivary flow in such patients is lifelong.
In addition to the general discomfort, hyposalivation may lead to difficulty in swallowing (dysphagia), chewing, and speaking; taste and smell changes (dysgeusia and dysosmia respectively) are also common . Without the lubricating effects of saliva, intraoral tissues may become friable and susceptible to irritation and inflammation (stomatitis), and dental prostheses are often poorly tolerated. Sequelae from hyposalivation also include erythema of the oral soft tissues, a furrowed and desiccated tongue, a shift in oral microflora toward cariogenic organisms (eg, Streptococcus mutans and lactobacillus species), hyposalivation-related dental caries, periodontal disease, and oral fungal infections (especially candidiasis) . Salivary dysfunction may also affect general health, because oral symptoms and dysgeusia and dysosmia may reduce appetite and alter food choices and may lead to nutritional compromise. For example, patients do not tolerate spicy, dry or hard foods. Xerostomia, the subjective complaint of a dry mouth, is virtually universal in patients who have undergone RT for head and neck cancer.
Prevention of salivary gland damage
Two drugs have been studied in the prevention of salivary gland damage during RT. One agent, pilocarpine (Salagen, MGI Pharma, Minneapolis, Minnesota) stimulates the salivary glands during radiation therapy, and has been suggested as a possible means of reducing damage to glands. Results of studies investigating the effects of pilocarpine (5 mg, 4 times/day) in patients receiving RT indicate that parotid function may be preserved, but not submandibular/sublingual function. Also, patients receiving pilocarpine have reported less frequent oral complaints . A second drug, amifostine (Ethyol, MedImmune, Gaithersburg, Maryland), is a sulfhydryl compound that acts as a potent scavenger of free radicals generated in tissues exposed to radiation, thus reducing radiation damage to DNA. Further, it appears to offer selective protection of normal cells, thereby not affecting tumor-directed treatment . Amifostine, when administered before radiation exposure, has been shown to significantly reduce the incidence of acute and chronic xerostomia , continuing its effect through 2 years following treatment . This agent has been approved by the US Food and Drug Administration (FDA) for management of RT-induced salivary gland dysfunction. Side effects include nausea, vomiting, and reversible hypotension that may be decreased with intramuscular administration.
Furthermore, studies have shown promise with more advanced techniques in cancer treatment. Salivary gland-sparing RT using three-dimensional RT techniques include intensity-modulated-radiation therapy (IMRT), with and without concomitant boost to the primary tumor site . With IMRT techniques, a focused high dose is administered directly and narrowly to the tumor. In effect, patients receive organ-sparing doses to the parotid gland contralateral to the tumor, and partial organ-sparing doses to the ipsilateral gland. This technique has allowed preservation of parotid gland tissue and reduction of xerostomia and hyposalivation, as well as improvement in quality of life following RT .
In addition, two studies have demonstrated successful surgical submandibular gland transfer to the submental space, placing the gland outside of the proposed radiation field. This procedure has allowed for appropriate shielding of the gland, resulting in a functioning gland after radiation .
Management of hyposalivation
Patients who experience hyposalivation must maintain excellent oral hygiene with fluoride agents and antimicrobials to prevent dental caries and oral infection. Periodontal disease may accelerate and caries may become rampant unless preventive measures are instituted. Frequent sipping of water and a moist diet are mandatory.
A variety of oral products have been developed to try to mimic saliva by temporarily lubricating the oral mucosa. These saliva substitutes and mouth-wetting agents (typically oral products containing hydroxyethylcellulose, hydroxypropylcellulose, or carboxymethylcellulose) are palliative agents that attempt to relieve the discomfort of xerostomia. Such products generally offer short-term relief of symptoms, and do not replace the antibacterial and immunologic protection of saliva .
Systemic saliva stimulants (sialogogues) stimulate saliva production from residual glands and produce greater relief than saliva substitutes. Conversely, such systemic stimulants will not work when there is no residual gland/function. Because water is the main component of saliva, the patient needs to drink water to be able to produce saliva. The use of sugarless gum or candies may also assist the stimulation of residual gland function.
Pilocarpine is a parasympathomimetic agent that causes stimulation of muscarinic/cholinergic receptors of salivary gland acinar cells. It is the only drug approved by the FDA for use as a sialogogue for radiation-induced xerostomia and hyposalivation. In doses of 5 to 10 mg three times per day, increased secretion of saliva occurs within 30 minutes after ingestion . For optimal results, the medication should be continued for 8 to 12 weeks , and may be used safely as maintenance therapy for longer treatment periods . The most common adverse effect at clinically efficacious doses is excessive sweating; less frequently, urinary frequency, lacrimation, and rhinitis are encountered.
Other sialogogues also appear to have efficacy in managing radiation-induced xerostomia and hyposalivation. Cevimeline (Evoxac, Daiichi Pharmaceuticals, Parsippany, New Jersey) 30 mg three times a day, is only approved for use in the management of Sjögren’s syndrome, but appropriate clinical trials are under way . Although cevimeline has greater selective affinity for M1 and M3 muscarinic (cholinergic agonist) receptors than pilocarpine, whether this can prove advantageous for treating RT-induced hyposalivation remains unclear. Bethanechol (Urecholine, Odyssey Pharmaceuticals, East Hanover, New Jersey) is a cholinergic agonist that has been shown to increase salivary flow with effects comparable to those of pilocarpine and cevimeline in clinical trials of patients who have xerostomia and hyposalivation following head and neck RT . Protocols for managing salivary dysfunction are outlined in Table 2 .
| If residual salivary function: | If no/minimal baseline salivary function: |
|---|---|
| Meticulous oral hygiene | Meticulous oral hygiene |
| Frequent sips of water | Frequent sips of water |
| Sugar free gums, mints, candy to stimulate salivary flow | |
| May have some benefit from mouth-wetting agents and salivary substitutes, such as Biotene Oral Balance Gel (Laclede Pharmaceuticals, Rancho Dominguez, California) and Mouth Kote (Parnell Pharmaceuticals, San Rafael, California) | Mouth-wetting agents and salivary substitutes, such as Biotene Oral Balance Gel and Mouth Kote |
| Systemic sialogogues | |
| Fluoride: 0.2% solution, 10–15 drops in custom vinyl tray used every night for 5 minutes after brushing and flossing. Brushing with prescription strength (1.1%) neutral sodium fluoride gel, such as PreviDent 5000+ (Colgate Oral Pharmaceuticals, New York, NY) at least once daily | Fluoride: 0.2% solution, 10–15 drops in custom vinyl tray used every night for 5 minutes after brushing and flossing. Brushing with prescription strength (1.1%) neutral sodium fluoride gel, such as PreviDent 5000+ at least once daily |
| Chlorhexidine gluconate 0.12%, hold and rinse one to two times per day to decrease oral flora | Chlorhexidine gluconate 0.12%, hold and rinse one to two times per day to decrease oral flora |
Oral infection
Acute oral infections of the mucosa (eg, bacterial, viral, and fungal), dentition/periapices, and periodontium may occur during and following cancer therapy because of exacerbation of latent or prior chronic infection, changes in oral flora that occur secondary to cancer treatment, or indirect damage to oral structures and tissues . Importantly, bacterial infections may not present with the usual swelling and suppuration if the patient is neutropenic during CT.
Bacterial infections may occur throughout therapy, and should be treated as soon as possible to reduce pain and the spread of infection. Compromised salivary function may also elevate the risk for oral infection. Chlorhexidine gluconate 0.12% is a broad-spectrum antimicrobial rinse with in vitro activity against gram-positive and gram-negative bacterial organisms and fungal species . It may be used to reduce gingivitis secondary to dental plaque and oral cariogenic microorganisms in patients who have undergone cancer treatment .
While undergoing CT, patients may become susceptible to viral infections caused by reactivation of a latent virus, such as herpes simplex virus (HSV) or varicella zoster virus . Patients who have been exposed to HSV may develop viral reactivation secondary to immunosuppression during CT . Patients receiving RT are not at increased risk of viral reactivation specifically related to therapy, although occasional instances of simultaneous oral HSV lesions occurring during therapy have been reported .
Candidiasis is a common clinical infection of the oropharynx in patients during and following CT or RT ( Fig. 2 ). A number of variables contribute to its clinical expression, including immunocompromised status, mucosal injury, and hyposalivation . Irradiated patients are particularly susceptible to candidal infection, as demonstrated by an increase in quantitative counts and rates for clinical infection during and following RT . RT results in hyposalivation, which creates a favorable environment for fungal overgrowth. Oral fungal infection is associated with adherent white plaques that often can be wiped off, inflammation, and erythema of oral tissues, and may result in a sore, burning mouth and taste change. Superficial oral fungal infections may be treated effectively with topical antifungal agents; however, persistent fungal infections or patients poorly compliant with topical therapy may require systemic antifungal medications (see the article by Treister and colleagues elsewhere in this issue).
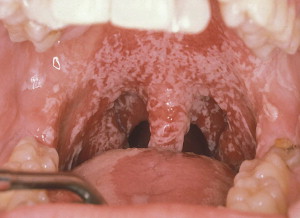
Late reactions
Chronic mucosal changes
The mucosal changes that occur with CT are usually acute, and healing occurs within weeks of cessation of cytotoxic CT.
In contrast, RT induces chronic changes of the oral mucosa as a result of hypovascular, hypocellular, and hypoxic changes . Types and severity of these changes are directly related to radiation dosimetry, including total dose, fraction size, and field size. Chronic mucosal sensitivity was reported by 43% of 65 respondents 1 year following RT . Chronic pain may result from permanent damage to oral tissues, including epithelial atrophy, fibrosis of connective tissues, neurologic sensitization, and/or neuropathy.
Mucosal atrophy and friability may predispose oral tissues to ulceration following trauma or injury . Soft-tissue necrosis may then ensue because of reduced vascularization of the tissue and poor wound healing , leading to pain that may be aggravated by secondary infection.
Caries
Dental caries risk increases in patients who have undergone RT ( Fig. 3 ). In addition to reducing salivary flow, RT may induce alterations in the composition of saliva, because decreases in secretory immunoglobulin A, pH, and bicarbonate concentration have been reported . Decreased concentrations of salivary antimicrobial proteins, reduced remineralizing potential of teeth, and a loss of buffering capacity contribute to dental caries. Furthermore, a shift toward a cariogenic flora has been observed in patients who have undergone RT, with increased colonization with Streptococcus mutans and lactobacillus species .
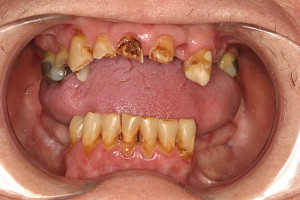
To decrease caries risk, optimal oral hygiene must be maintained. Hyposalivation should be managed by use of salivary stimulants, reinforcing frequent sips of water, and palliated with salivary substitutes or stimulants. Caries resistance can be enhanced with use of prescription-strength topical fluorides or remineralizing agents, which are high in calcium phosphate and fluoride . Custom vinyl carrier trays can be made to extend 1 to 2 mm beyond the gingival margins of teeth, thereby allowing these products extended contact time with tooth structure and increased uptake into the enamel. Compliance with use of fluoride trays must be reinforced , and those not able to effectively comply with use of fluoride trays should be instructed to use high-potency brush-on fluoride gels (such as 1.1% neutral sodium fluoride gel), and rinses .
Post-radiation soft-tissue and bone osteonecrosis
Post-radiation osteonecrosis (PRON) is another well-recognized complication of head and neck RT ( Fig. 4 A, B). Loss of bone vitality occurs secondary to injury to osteocytes, osteoblasts, and osteoclasts, as well as relative hypoxia caused by reduction in vascular supply . These changes can lead to a reduced capacity of soft tissue and bone to recover from injury, predisposing to soft-tissue necrosis and osteonecrosis . Soft-tissue necrosis may involve any mucosal surface in the mouth, although nonkeratinized surfaces appear to be at moderately higher risk. Presenting clinical features of PRON include painful or painless exposure of necrotic bone or sequestra, paresthesia or anesthesia, secondary infection, and sinus tract or fistula formation . With progressive destruction of bone, pathologic fracture may occur.
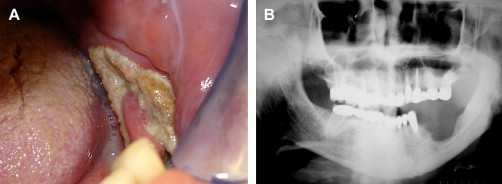
The risk for PRON is directly related to radiation technique, dose, and volume of tissue irradiated. Patients who have received high-dose radiation (>60 Gy) to the head and neck are at lifelong risk for PRON, with an overall risk of approximately 4% to 15% with standard fractionation ; the risk increases significantly after 66 Gy . The risk for PRON is lifelong, may occur many years after RT, and does not decrease with time . Delivering higher doses with moderately accelerated or hyperfractionated schedules could reduce the risk of PRON . A further decrease in risk may be achieved using IMRT, because only a small volume of alveolar bone is exposed to high radiation doses, although boost technique results in higher total doses to primary tumor that may include bone . PRON more frequently involves the mandible versus the maxilla, likely because of greater bone density and unilateral vascular supply to each half of the mandible , and an increased risk in PRON has been described when more than half of the mandible is exposed to high dose radiotherapy .
Prevention of PRON begins with comprehensive assessment and oral care before head and neck RT (see the article by Brennan and Lockhart elsewhere in this issue). Comprehensive management of PRON includes removal of bony sequestrae and use of antibacterial rinses (eg, chlorhexidine gluconate), topical antibiotics (eg, tetracycline rinse), or systemic antibiotics (eg, penicillin or clindamycin) to facilitate wound resolution . In addition, patients should be followed and advised regarding minimization or elimination of trauma and avoidance of use of removable dental prosthesis if the denture-bearing area is within the osteonecrotic field, adequate nutritional intake, and discontinuation of tobacco and alcohol. Analgesics for pain control are often needed.
In cases associated with pain and progression, hyperbaric oxygen therapy (HBO) is recommended for management of PRON , although some studies show it may be of limited benefit . Numerous uncontrolled studies have shown recovery rates of 15% to 45% with HBO alone, and 20% to 90% with HBO combined with surgery . Further, a Cochrane database systematic review of controlled trials found that following HBO for PRON, there was an improved probability of achieving complete mucosal cover, bony continuity, and healing of tooth sockets following extraction compared with no HBO treatment. The probability of recovery was much greater following hemimandibulectomy and surgical flap placement following HBO compared with no treatment . In some circumstances, HBO may also be considered prophylactically if surgery is required in the area susceptible to PRON. HBO increases oxygenation of irradiated tissue, promotes angiogenesis, and enhances osteoblast repopulation and fibroblast function. HBO is usually prescribed as 20 to 30 dives at 100% oxygen and 2 to 2.5 atmospheres of pressure. If surgery is needed, 10 dives of postsurgical HBO therapy are recommended .
Dental implants after radiation
The literature is inconclusive regarding dental implant placement following ionizing radiation to the head and neck. Extraoral and intraoral implant survival is significantly lower in irradiated compared with non-irradiated bone at doses of at least 50 Gy, primarily because of the long-term effects of reduced vascularization compromising the implantation site . The lower survival in irradiated bone compared with non-irradiated bone supports the argument for preventive HBO treatment , which has improved implant survival rates in all areas subjected to radiation therapy . Some authors have recommended that HBO therapy should be used as adjunctive therapy when the implant site is irradiated with more than 50 Gy and shows clinical signs of radiation damage, in order to enhance osseointegration and diminish healing complications such as PRON . No significant differences in implant survival rates have been reported when comparing timing of implant placement less than 12 months after radiation therapy to greater than 12 months after treatment . Irradiation with implants already placed in the radiation field may be encountered; it appears that implant failures increase with longer follow-up .
Speech and mastication
Treatment of head and neck cancers can create surgical defects and fibrosis that result in severe oral dysfunction and facial disfigurement. Surgical maxillectomy procedures produce defects in the hard or soft palate. Rehabilitation is accomplished with an obturator prosthesis, which restores function in speech, mastication, and deglutination . Tongue and mandibular resections affect tongue mobility and mandibular movement. This may result in impaired speech, difficulty with mastication, dysphagia, deviation of the mandible during functional movements, poor control of salivary secretions, and disfigurement. Tongue function may be restored with myocutaneous or free flaps, and bone grafts may be used for mandibular discontinuity defects . Ultimately, speech and physical therapy are also required for complete rehabilitation.
Nutrition, taste and smell impairment
Taste and smell alterations are generally transient after CT.
Taste is altered as an early response to RT, and may present as a reduction in taste sensitivity (hypogeusia), an absence of taste sensation (ageusia), or a distortion of normal taste (dysgeusia) . During a curative dose of RT, taste function for bitter flavor becomes impaired during the first week and gradually worsens. Taste loss may begin with radiation doses of 20 Gy, and with 30 Gy all taste qualities are affected. Ninety percent of all patients experience a loss of taste when the cumulative dose has reached 60 Gy . Taste loss may be caused by direct radiation damage to the cells in the taste buds or innervating fibers . Histologically, the architecture of the taste buds is almost completely destroyed at therapeutic RT levels . These damaged cells usually regenerate within 4 month of treatment, so taste returns to normal or near normal levels within 1 year post-RT ; however, permanent alteration may result, which may be related to damage to the neural component of taste . Zinc sulfate, 220 mg two times per day, has been reported to help with recovery of taste disturbances .
Alterations of smell may also develop with RT when the olfactory mucosa is exposed to radiation. This occurs more commonly in the course of treatment for nasopharyngeal carcinoma and maxillary antrum cancers. During RT, smell acuity reduces (hyposmia), and an altered recognition of odor (dysosmia) develops. Similar to taste, smell changes are often transient, and complete recovery often occurs within 1 year after cessation of treatment . The use of saline nasal sprays are sometimes helpful.
Taste and smell impairments greatly impact the patient’s quality of life and, coupled with other RT-related comorbidities such as hyposalivation and dysphagia, reduce food enjoyment, and may affect the nutritional status and overall health of the patient . Nutritional counseling may be required after therapy; maintenance of appropriate caloric and nutrient intake should be emphasized. Consideration must also be given to moisture and texture of foods.
Trismus and other musculoskeletal presentations
Trismus may develop if the masticatory muscles or the temporomandibular joint (TMJ) are involved in cancer treatment . Surgical treatment may produce scar tissue, which reduces mouth opening because of scar contraction and fibrosis of the masticatory muscles. Additionally, RT may induce fibrosis and atrophy in the masticatory muscles or TMJ as a late radiation effect in 5% to 38% of patients, typically developing 3 to 6 months after RT . Trismus may increase morbidity because the limitation in opening interferes with oral hygiene, speech, nutritional intake, examination of the oropharynx, and dental treatment.
Masticatory or cervical muscle pain may result from tumor invasion or cancer therapy. This muscle dysfunction may cause pain, fibrosis, and limited range of movement, often becoming a lifelong problem. Patients who have undergone neck dissection have reported neck tightness and cervical muscle pain , with greater pain levels reported following modified radical compared with selective neck dissection .
In patients who present with trismus or muscle pain, the goal is to restore range of motion and to alleviate pain and dysfunction. To treat trismus, exercises to increase mouth opening and improve mandibular mobility include the use of dynamic bite openers (Therabite, Atos Medical Inc, West Allis, Wisconsin), rubber plugs, and varying numbers of tongue blades stacked together, which increase mouth opening with passive stretching. Furthermore, pentoxifylline is a methylxanthine derivative that has immunomodulatory properties that down-regulates of certain cytokines, some of which may have a significant role in the pathogenesis of radiation-induced fibrosis . Pilot data have shown that pentoxifylline administered to patients who have RT-induced fibrosis has resulted in a significant increase in mandibular range of motion . Once trismus is established, only a limited increase in range of movement can be achieved; however, with no intervention, changes are often progressive. Involvement of other health professionals to establish muscle pain control and restore function may be warranted. Regardless of the approach, patient compliance and perseverance are essential for success, because dramatic results are not achieved immediately .
Neuropathic pain and neurotoxicity
Surgery for cancer commonly results in acute orofacial pain, and may lead to painful post-traumatic neuropathy. Neuropathic pain has been reported in patients following resection of the mandible , 2 to 5 years post maxillectomy , and 1 month to 27 years following neck dissection procedures .
Chemotherapy-induced peripheral neuropathy (CIPN) typically resolves with or without treatment, although in some patients it may evolve into a chronic painful condition resulting in sensory and motor changes . Prevalence during treatment is variable with agents, the intensity of treatment (dose intensity and cumulative dose), other ongoing therapies (such as surgery and RT), age of the patient, and the use of combinations of CT agents . Commonly used neurotoxic agents such as the taxanes (paclitaxel, docetaxel), vinca alkaloids (vincristine, vinblastine), platinum-based compounds (cisplatin, oxaliplatin), thalidomide, and bortezomib appear to be the most responsible for precipitating CIPN .
RT may also cause neural damage resulting in neuropathic pain, with higher doses causing increased morbidity (see the article by Clark and Ram elsewhere in this issue).
Dentofacial abnormalities
Altered dental growth and development is a frequent complication for children who are long-term cancer survivors after having received CT or RT. Children treated at younger than 12 years often exhibit developmental disturbances, such as alteration in the size and shape of teeth, delayed eruption of teeth, and other craniofacial developmental abnormalities . Abnormal tooth formation manifests as decreased crown size, shortened and conical-shaped roots, and microdontia; on occasion, complete agenesis may occur. Eruption of teeth can be delayed, including increased frequency of impacted maxillary canines. Shortened root length is associated with smaller alveolar processes, which in turn leads to decreased occlusal vertical dimension. Additionally, treatment-related injury to maxillary and mandibular growth centers can compromise full maturation of the craniofacial complex. If bilateral RT is administered, changes tend to be symmetric, and the effect is not always clinically evident. Cephalometric analysis typically is necessary to delineate the scope of the condition. When unilateral RT is provided, asymmetrical changes are usually obvious.
Recurrent disease and second primary cancers
Patients who have a history of head and neck cancers have an increased risk of developing recurrences or second primary cancers. The rate of second primary cancers among patients who have head and neck cancer is relatively high when compared with that of other cancers . Locoregional tumor failure is reported to occur in 16% to 34% of all oral cancer patients , with the most common sites being the tongue, tonsils, and pharynx . Death from uncontrolled locoregional or distant disease occurs in about 35% of the patients who have recurrent disease, and another 15% die from non-oral malignancies .
The increased risks of second primary tumors are most prominent among patients who are 60 years or younger, with no gender or race predilection . Tobacco smoking and alcohol drinking each contribute to the risk of second cancers, with the effects of smoking more pronounced than those of alcohol . The risk of a second primary cancer rises with increasing intensity and duration of smoking.
Because of the significant risk of developing recurrent disease or second primary cancer, patients who have a history of head and neck cancer must undergo close surveillance. In the oral cavity, surgery and radiation treatment may induce scar formation and irregular anatomy of structures, thereby complicating evaluation of intraoral tissues. Patients must undergo routine follow-up with an oncologist to include careful examination of all structures of the head and neck, as well as annual chest radiographs.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


