Orofacial pain and altered nerve sensation may be the initial sign of oropharyngeal or nasopharyngeal cancer. This article focuses on the most common orofacial pain conditions and neurosensory alterations that affect cancer patients, such as neuropathic pain, muscle spasm or contractures, mucositis, and increased or decreased sensory discrimination in the affected area. The various pharmacotherapeutic modalities for cancer pain management ranging from non steroidal anti-inflammatory drugs (NSAIDs) for mild pain to opioids for severe pain are discussed in detail.
Orofacial pain and altered nerve sensation may be the initial sign of oropharyngeal or nasopharyngeal cancer. This article focuses on the most common orofacial pain conditions and neurosensory alterations that affect cancer patients, such as neuropathic pain, muscle spasm or contractures, mucositis, and increased or decreased sensory discrimination in the affected area. The various pharmacotherapeutic modalities for cancer pain management ranging from nonsteroidal anti-inflammatory drugs (NSAIDs) for mild pain to opioids for severe pain are discussed in detail.
Nociceptive versus neuropathic pain
Pain is described as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage ( http://www.iasp-pain.org .iasp-pain.org). Dysesthesia is defined as an unpleasant abnormal sensation, whether spontaneous or evoked. Allodynia is defined as pain caused by a stimulus which does not normally provoke pain. Pain can arise from a variety of structures in the head and neck region, such as the teeth, muscles, temporomandibular joint, ear, paranasal sinuses, pharynx, and so forth. Pain may also be referred from one region of the head and neck to another, as is commonly encountered in myofascial pain. The trigeminal nerve provides sensory innervation for the face, teeth, and oral soft tissues, and is the main nerve involved with pain sensations of the head and neck region. Other nerves that are infrequently responsible for pain transmission in this region include the glossopharyngeal and occipital nerves.
Regardless of its causation or site of origin, pain is typically divided into nociceptive and neuropathic pain. Nociceptive pain is caused by actual tissue injury and inflammation. In cancer this may develop as the tumor invades or enlarges and generates inflammatory mediators such as prostaglandins and leukotrienes, especially when bone is involved . The critical role of prostaglandins in the inflammatory cascade is supported by the efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) as one of the treatment modalities in cancer pain management (see Non-opioid analgesics and NSAIDs, below). Patients with nociceptive pain usually require a shorter time and less complicated analgesic regimens to achieve stable pain control. Nociceptive pain implies that the nervous system is functioning normally and is responsive to all forms of analgesics, including opioids. Assuming the patient is opioid-naïve, he usually requires lower starting opioid doses to achieve adequate pain control versus those who have neuropathic pain .
In contrast, neuropathic pain is much more difficult to control with analgesic medications, especially when complicated with comorbid psychological distress. Neuropathic pain results when the nociceptors act in an abnormal fashion because of sustained or repetitive stimulation or injury to the nerve. Neuropathic pain is defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system , or as pain caused by a lesion of the peripheral or central nervous system (or both) manifesting with sensory symptoms and signs . It is common for patients who have cancer to suffer from a combination of both nociceptive and neuropathic pain ; however, it has been demonstrated that approximately 50% of pain in these patients can be categorized as exclusively or partly neuropathic . Pain is not only caused by cancer itself—20% of patients suffer from treatment-related pain .
Pain as the first sign of cancer
Orofacial pain may be the initial symptom of oropharyngeal or nasopharyngeal cancer, or other systemic malignancies such as multiple myeloma, leukemia, or lymphomas; these cancers may present as gingival infiltrates, loose teeth, bleeding gingiva, or masses in the oral cavity. Pain can also be referred to the orofacial region from cancer originating in areas adjacent to the head and neck. Cancer must always be considered in the differential diagnosis of a patient who has a chronic unexplained or unresponsive pain process (eg, atypical toothache, trismus, or even a temporomandibular disorder) , even when clinical examination, plain radiography, CT, and MRI are negative . The risk of cancer increases with age, and in any patient who has had a previous diagnosis of malignancy . A 2006 retrospective study of 1412 patients who had oral cancer showed that pain was the first clinical sign of oral cancer in 19% of the cases ( Table 1 ). Orofacial pain has also been reported to be one of the earliest indicators of recurrent cancer.
| Type of pain complaint | Percent of sample |
|---|---|
| Sore throat | 37.6% |
| Tongue pain | 14.0% |
| Mouth pain | 12.9% |
| Pain when swallowing | 11.1% |
| Dental pain | 5.9% |
| Earache | 5.9% |
| Pain in the palate | 4.1% |
| Burning mouth | 3.3% |
| Gingival pain | 2.2% |
| Pain when chewing | 1.1% |
| Neck pain | 1.1% |
| Facial pain | 0.7% |
Pain prevalence and oropharyngeal and nasopharyngeal cancer
A 2002 National Institutes of Health (NIH) State of the Science panel found that pain is one of the most common side effects of cancer and cancer treatments . A 2007 study of hospitalized cancer patients in Norway found that 52% experienced cancer-related pain, with a mean pain level of 4 on a 10-point scale in spite of their medications . Similarly, in another study, 52% of cancer patients hospitalized at the National Cancer Institute of Milan were found to have pain, with 50% attributable to their cancer surgery and 29% caused by the tumor mass itself .
What makes oropharyngeal and nasopharyngeal cancer painful?
The most common cause of cancer-related pain in the head and neck region is secondary to neural compression. The pain seen with an oral, pharyngeal, or nasopharyngeal cancer generally occurs from direct perineural invasion of peripheral nerves or spread of the lesion intracranially. In bony cancers more so than soft tissue cancers, the destructive biological process associated with tumor invasion causes inflammatory reactions resulting in deep bone pain. Chong and colleagues examined the various pathways of intracranial spread of nasopharyngeal cancer in 114 consecutive patients using CT scans and T1-, T2-weighted, contrast-enhanced MRI images. They found that 31% of patients had middle cranial fossa involvement, 25% of patients exhibited cavernous sinus infiltration, and 5% of patients showed only dural thickening. Of those who had middle cranial fossa spread, the most common routes were through the foramen ovale (34%), skull base (17%), foramen lacerum (17%), sphenoid sinus (17%), and a combination of foramina ovale and lacerum (14%). Intracranial spread of cancer can also occur in the absence of any pain. Su and Lui examined the correlation between clinical neurologic symptoms and proven trigeminal perineural invasion by CT and MRI in 110 patients who had newly diagnosed nasopharyngeal carcinoma. Facial pain or paresthesia was noted in 24% of cases; however, 54% of the patients had radiologically demonstrable perineural tumor invasion of the trigeminal nerve.
Cancer pain can be controlled in more than 70% of patients using a simple opioid-based regimen . Because of nerve sensitization, neuronal suppression with opioids or NSAIDs alone is insufficient, and therefore anticonvulsants have become an essential aspect of treatment. In injured neurons, among the many identified changes that cause inappropriate nerve firing in response to subthreshold stimuli or an increased response to suprathreshold stimuli is an up-regulation of highly responsive sodium channels. These channels accumulate at the site of nerve injury along the length of axons, and are the source of ectopic and spontaneous action potential discharges. Anticonvulsants are more effective than opioids because their primary mode of action is to inhibit pain by blocking the highly responsive sodium channels. Other receptors have been identified that are up-regulated or activated in neuropathic pain, including the P2X receptors, vanilloid receptors, cannabinoid receptors, and proton receptors, and are all potential targets of therapies. Currently, however, there are no drug therapies available that specifically and effectively target these receptors.
Prominent oral and maxillofacial side effects of cancer and cancer treatments
Patients treated by surgical excision, radiotherapy, and chemotherapy for cancers frequently experience problematic orofacial symptoms . The most substantial chronic oral side effects include dysfunction (trismus/contractures of the jaw muscles), pain (mucositis and neuropathic), and oral sensory alterations (numbness and sensory distortions). Patients who have hematologic malignancies that are treated with allogenic hematopoietic stem cell transplantation may develop oral pain secondary to chronic graft-versus-host disease (cGVHD) (see the article by Schubert and Correa elsewhere in this issue) .
Secondary spasm/contracture
Persistent fibrotic contracture of the jaw muscles and trismus are significant side effects of radiotherapy and cancer surgery . This can make it nearly impossible to maintain appropriate dental hygiene and receive dental treatment. The prevalence of trismus after head and neck oncology treatment ranges from 5% to 38% . Ng and Wei reported that in 41 patients who had undergone maxillary swing surgery to treat nasopharyngeal carcinoma, 71% developed severe trismus, defined as an interincisal opening less than 25 mm. One report suggested that the most decisive factor in whether trismus develops or not is probably the inclusion of the medial pterygoid muscles in the treatment portal during surgery or radiotherapy . When cancers of the base of tongue, hypopharynx, esophagus, or larynx are treated with radiation therapy, stricture and fibrosis contribute to long-term swallowing complications that require intensive physical therapy, and often long-term parenteral nutritional support.
Treatment of surgical or radiation-induced persistent postoperative trismus/contracture generally has a poor outcome. The primary approach includes: (1) stretching under sedation (this distinguishes trismus from a permanent muscle fibrosis or contracture), and (2) weekly office and daily home use of passive jaw stretching exercises . The Therabite (Astos Medical, Wisconsin) ( Fig. 1 ) is a commercially available stretching device shown to improve the maximal interincisor opening in patients who have post surgical trimus by an average of 10 mm . A low-cost alternative to the Therabite includes the use of two stacks of tongue blades inserted between the posterior teeth to increase the size of mandibular opening ( Fig. 2 ). Buchbinder and colleagues compared Therabite with tongue blade therapy and unassisted jaw opening exercises in post-irradiated patients, and found that a greater sustained increase in mouth opening was achieved with the Therabite. Most importantly, stretching exercises need to be implemented early and aggressively in the treatment period to maintain maximum opening and jaw mobility . Patient compliance and perseverance are critical factors for successful treatment outcome despite the choice of therapy.
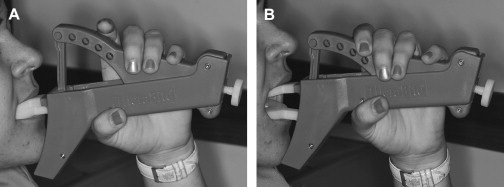
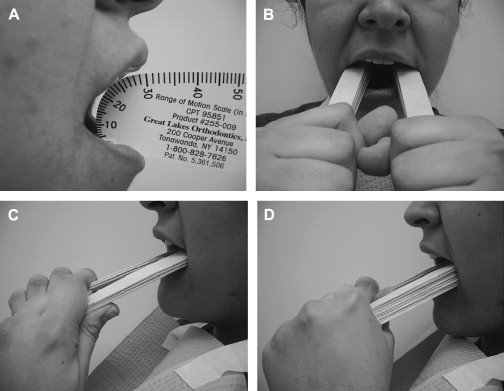
When the cancer or treatment is causing spastic reaction in the jaw closing musculature, botulinum toxin (BoTN) injection into the involved muscles can be effective . A recent study described the use of BoTN-A (a particular form of BoTN) for treating secondary painful spasms of the neck musculature associated with radiotherapy . The BoTN-A injections were administered into the most affected sternocleidomastoid muscles in one or two locations, with four out of six patients achieving durable relief.
Oral mucositis
Mucositis is a common painful oral complication of cytotoxic chemotherapy and head and neck chemoradiotherapy, and when severe, is a frequent reason for unplanned breaks in treatment (see the article by Lalla and colleagues elsewhere in this issue). Treatment of mucositis has until recently been largely palliative; however, palifermin, a keratinocyte growth factor, has been shown in a Phase III clinical trial to be effective in reducing the incidence and duration of severe mucositis in patients treated with high-dose conditioning regimens followed by autologous stem cell rescue . The pain secondary to mucositis can be partially managed by prescribing a number of topical agents. Benzydamine hydrochloride, a NSAID, has been shown in multicenter, double-blind controlled studies to reduce mucositis and pain in patients who had head and neck cancer ; however, these results have been inconsistent, and this agent is not approved by the US Food and Drug Administration (FDA) or available in the United States. Other approaches to pain management in mucosistis patients involve the use of topical anesthetics such as 20% benzocaine in Orajel (Colgate Orabase, New York, New York), 2% to 4% viscous lidocaine and sucralfate suspension . Topical morphine may be effective in reducing the duration of pain and functional impairment associated with mucositis . Although the mechanism is unclear, low-energy helium-neon laser appears to be effective in reducing the duration and severity of mucositis; further research is needed .
Neuropathic pain
Neuropathic pain secondary to tumor invading the peripheral or central nervous system or as a sequela of treatment may affect as many as 25% of patients . Surgical interventions that stretch or transect nerves result in higher incidences of long-term neuropathic pain sequelae. Manfredi and colleagues examined cancer patients who had neural lesions resulting in neuropathy in 103 patients. They that the most frequent sites of neurological injury were nerve roots, spinal cord and cauda equina, brachial and lumbosacral plexus, and peripheral nerves, with no cases caused by brain injury. Chemotherapy-induced peripheral neuropathy is a common side effect observed following exposure of patients to vinca alkaloids, taxanes, platinum-derived compounds, suramin (a growth factor antagonist), thalidomide, and most recently bortezomib (a proteasome inhibitor) therapy. Incidence reports vary widely from 10% to 100% . Chemotherapy-induced neuropathy typically affects the small myelinated and unmyelinated nerve fibers. Peripheral neuropathy is occasionally associated with cGVHD. The management of neuropathic pain is complex and involves the use of various medications ranging from anticonvulsants and antidepressants to local anesthetics and N-methyl-D-aspartate receptor blockers (see sections below).
Oral neurosensory alterations
Neurosensory abnormalities (reduced, abnormal, or increased sensations in a local area) that interfere with chewing and swallowing are common in patients following treatment for oral and nasopharyngeal cancer. Patients treated with surgery or radiotherapy for oral and pharyngeal cancer exhibit decreased sensory discrimination that contributes to long-term masticatory and swallowing complications, occasionally resulting in silent and non-silent aspiration . Chronic taste changes, especially following radiotherapy, may affect diet and nutrition. Zinc sulfate has been reported anecdotally to improve the taste alterations in these patients; however, a recent double-blind, placebo-controlled trial of zinc sulfate in patients undergoing radiation therapy for the oropharynx showed that zinc sulphate was ineffective in preventing taste changes .
Prominent oral and maxillofacial side effects of cancer and cancer treatments
Patients treated by surgical excision, radiotherapy, and chemotherapy for cancers frequently experience problematic orofacial symptoms . The most substantial chronic oral side effects include dysfunction (trismus/contractures of the jaw muscles), pain (mucositis and neuropathic), and oral sensory alterations (numbness and sensory distortions). Patients who have hematologic malignancies that are treated with allogenic hematopoietic stem cell transplantation may develop oral pain secondary to chronic graft-versus-host disease (cGVHD) (see the article by Schubert and Correa elsewhere in this issue) .
Secondary spasm/contracture
Persistent fibrotic contracture of the jaw muscles and trismus are significant side effects of radiotherapy and cancer surgery . This can make it nearly impossible to maintain appropriate dental hygiene and receive dental treatment. The prevalence of trismus after head and neck oncology treatment ranges from 5% to 38% . Ng and Wei reported that in 41 patients who had undergone maxillary swing surgery to treat nasopharyngeal carcinoma, 71% developed severe trismus, defined as an interincisal opening less than 25 mm. One report suggested that the most decisive factor in whether trismus develops or not is probably the inclusion of the medial pterygoid muscles in the treatment portal during surgery or radiotherapy . When cancers of the base of tongue, hypopharynx, esophagus, or larynx are treated with radiation therapy, stricture and fibrosis contribute to long-term swallowing complications that require intensive physical therapy, and often long-term parenteral nutritional support.
Treatment of surgical or radiation-induced persistent postoperative trismus/contracture generally has a poor outcome. The primary approach includes: (1) stretching under sedation (this distinguishes trismus from a permanent muscle fibrosis or contracture), and (2) weekly office and daily home use of passive jaw stretching exercises . The Therabite (Astos Medical, Wisconsin) ( Fig. 1 ) is a commercially available stretching device shown to improve the maximal interincisor opening in patients who have post surgical trimus by an average of 10 mm . A low-cost alternative to the Therabite includes the use of two stacks of tongue blades inserted between the posterior teeth to increase the size of mandibular opening ( Fig. 2 ). Buchbinder and colleagues compared Therabite with tongue blade therapy and unassisted jaw opening exercises in post-irradiated patients, and found that a greater sustained increase in mouth opening was achieved with the Therabite. Most importantly, stretching exercises need to be implemented early and aggressively in the treatment period to maintain maximum opening and jaw mobility . Patient compliance and perseverance are critical factors for successful treatment outcome despite the choice of therapy.
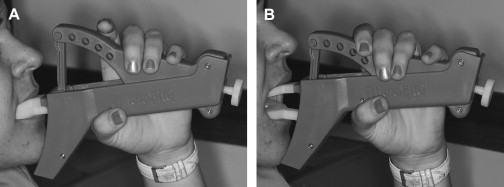
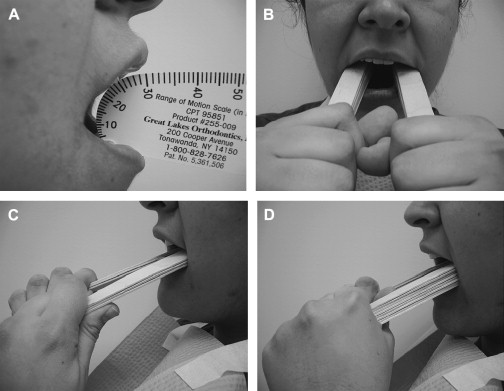
When the cancer or treatment is causing spastic reaction in the jaw closing musculature, botulinum toxin (BoTN) injection into the involved muscles can be effective . A recent study described the use of BoTN-A (a particular form of BoTN) for treating secondary painful spasms of the neck musculature associated with radiotherapy . The BoTN-A injections were administered into the most affected sternocleidomastoid muscles in one or two locations, with four out of six patients achieving durable relief.
Oral mucositis
Mucositis is a common painful oral complication of cytotoxic chemotherapy and head and neck chemoradiotherapy, and when severe, is a frequent reason for unplanned breaks in treatment (see the article by Lalla and colleagues elsewhere in this issue). Treatment of mucositis has until recently been largely palliative; however, palifermin, a keratinocyte growth factor, has been shown in a Phase III clinical trial to be effective in reducing the incidence and duration of severe mucositis in patients treated with high-dose conditioning regimens followed by autologous stem cell rescue . The pain secondary to mucositis can be partially managed by prescribing a number of topical agents. Benzydamine hydrochloride, a NSAID, has been shown in multicenter, double-blind controlled studies to reduce mucositis and pain in patients who had head and neck cancer ; however, these results have been inconsistent, and this agent is not approved by the US Food and Drug Administration (FDA) or available in the United States. Other approaches to pain management in mucosistis patients involve the use of topical anesthetics such as 20% benzocaine in Orajel (Colgate Orabase, New York, New York), 2% to 4% viscous lidocaine and sucralfate suspension . Topical morphine may be effective in reducing the duration of pain and functional impairment associated with mucositis . Although the mechanism is unclear, low-energy helium-neon laser appears to be effective in reducing the duration and severity of mucositis; further research is needed .
Neuropathic pain
Neuropathic pain secondary to tumor invading the peripheral or central nervous system or as a sequela of treatment may affect as many as 25% of patients . Surgical interventions that stretch or transect nerves result in higher incidences of long-term neuropathic pain sequelae. Manfredi and colleagues examined cancer patients who had neural lesions resulting in neuropathy in 103 patients. They that the most frequent sites of neurological injury were nerve roots, spinal cord and cauda equina, brachial and lumbosacral plexus, and peripheral nerves, with no cases caused by brain injury. Chemotherapy-induced peripheral neuropathy is a common side effect observed following exposure of patients to vinca alkaloids, taxanes, platinum-derived compounds, suramin (a growth factor antagonist), thalidomide, and most recently bortezomib (a proteasome inhibitor) therapy. Incidence reports vary widely from 10% to 100% . Chemotherapy-induced neuropathy typically affects the small myelinated and unmyelinated nerve fibers. Peripheral neuropathy is occasionally associated with cGVHD. The management of neuropathic pain is complex and involves the use of various medications ranging from anticonvulsants and antidepressants to local anesthetics and N-methyl-D-aspartate receptor blockers (see sections below).
Oral neurosensory alterations
Neurosensory abnormalities (reduced, abnormal, or increased sensations in a local area) that interfere with chewing and swallowing are common in patients following treatment for oral and nasopharyngeal cancer. Patients treated with surgery or radiotherapy for oral and pharyngeal cancer exhibit decreased sensory discrimination that contributes to long-term masticatory and swallowing complications, occasionally resulting in silent and non-silent aspiration . Chronic taste changes, especially following radiotherapy, may affect diet and nutrition. Zinc sulfate has been reported anecdotally to improve the taste alterations in these patients; however, a recent double-blind, placebo-controlled trial of zinc sulfate in patients undergoing radiation therapy for the oropharynx showed that zinc sulphate was ineffective in preventing taste changes .
Analgesic-based pharmacologic therapy of cancer pain
When pain is severe, opioid analgesics are necessary, regardless of whether the etiology is neuropathic or nociceptive. Non-opioid analgesics and other adjunctive medications are effective when the pain is less severe, and are often used in combination with opioids for improved analgesic effects. Although this article focuses on those being treated for pain in an outpatient fashion, a brief overview of hospital-based cancer pain management with opioid therapy is also included, because some patients may be managed similarly in the home setting.
Opioid therapy for cancer pain
In recent years Cochrane reviews and meta-analyses have concluded that sufficient evidence exists to state that morphine , hydromorphone , and methadone are effective for managing cancer pain. Although not surprising, these reviews also state that there is a lack of high quality evidence. Although the reviewers were unable to conclude which opioid is the ideal starting agent, some common opioid protocols are provided ( Table 2 ).
| Opioid | Approximate dose for equivalent analgesia | Half-life (hours) | Peak (hours) | Duration (hours) | Indication |
|---|---|---|---|---|---|
| Codeine | 130 mg IM | 2–4 | 1 | 4–6 | Mild to moderate pain |
| 200 mg PO | 2–4 | 1–2 | 4–6 | ||
| Dihydrocodeine | 50–75 mg PO | 2–4 | 1 | 4–6 | Mild to moderate pain |
| Hydrocodone | 15 mg PO | 2–4 | 1 | 4–6 | Mild to moderate pain |
| Oxycodone | 30 mg PO | 2–3 | 1 | 3–6 | Mild to moderate or severe pain |
| Morphine | 10 mg IM/IV | 2–4 | 0.5–1 | 4–6 | Moderate to severe pain |
| 20–60 mg PO | 2–4 | 2 | 4–6 | ||
| Hydromorphone | 1.5 mg IM | 2–3 | 0.5–1 | 4–6 | Moderate to severe pain |
| 7.5 mg PO | 2–3 | 1–2 | 4–6 | ||
| Fentanyl | 0.1 mg IV | 3–4 | 0.25 | 0.5–2 | Moderate to severe pain |
An excellent set of treatment guidelines with a step-by-step algorithm was developed for hospital and hospice cancer patients to guide cancer pain management . Briefly, these guidelines suggest that: (1) opioid-naïve cancer patients who have severe pain should receive rapidly escalating doses of short-acting opioids, (2) constipation prevention should be included because this adverse effect inevitably develops, and (3) opioid-naïve patients should also be given a nonopioid analgesic such as ibuprofen or acetaminophen to supplement their opioid medication. The most common initial opioid medications are morphine, hydromorphone, fentanyl, or oxycodone. After the initial response and treatment of acute pain in the hospital, the patient should have a comprehensive reassessment of pain symptoms and signs, and routine follow-up should be scheduled at least every 3 months, on an outpatient basis, depending on patient conditions and institutional standards.
Endogenous opioid-induced tolerance to exogenous opioids
It is well-established that opioids are generally ineffective as primary agents in managing neuropathic pain. One explanation is that the patient is already tolerant to opioids, even before they are prescribed. Neuropathic pain activates chronic endogenous opioid release, potentially inducing levels of tolerance without exposure to exogenous opioids . This may explain why some patients, especially those who have neuropathic pain, do not respond at standard doses, whereas other opioid-naïve patients typically respond. The solution is to simply raise the opioid dose until an analgesic response is seen. In fact, data clearly suggest that if clinicians carefully follow the World Health Organization (WHO) guidelines ( Table 3 ) escalating from non-opioid analgesics to moderate strength opioids, and then moving to stronger opioids when pain control is not adequate, it will result in improvement in all patients regardless of initial pain diagnosis . When using opioids, the issue of tolerance is an area of concern. Conventional wisdom is that as tolerance develops, the opioid dose must be increased or a different opioid must be substituted. Methadone is commonly selected as the opioid of choice, because patients are less likely to develop rapid tolerance .



