The myelosuppressive and mucosal-damaging consequences of cancer and cancer therapies place patients at high risk for developing infectious complications. Bacterial, fungal, and viral infections are all commonly encountered in the oral cavity, contributing to both morbidity and mortality in this patient population. Prevention, early and definitive diagnosis, and appropriate management are critical to ensure optimal treatment outcomes. With the majority of cancer patients treated as outpatients in the community setting, oral health care professionals play an important role in managing such infectious complications of cancer therapy.
Patients who have cancer are at risk both for newly acquired infections and, more often, from exacerbation of chronic infections or reactivation of latent viruses. During cancer therapy, the neutrophil count may be low, placing patients at risk for bacterial infections (in particular, odontogenic infections) . Compromised lymphocyte function places them at risk for infections in which immunity is mediated by T-cells, especially fungal and viral infections. The presence of chemotherapy-induced mucositis may act as a portal of entry for oral bacteria into the blood stream, leading to bacteremia and sepsis. It is during this period that oral infections have a high morbidity and even mortality rate for patients.
After cancer therapy, and for the rest of their lives, patients may continue to experience some degree of immune compromise and suboptimal lymphocyte function; therefore, the oral health care specialist should have a high index of suspicion for oral lesions that do not heal, as they may be infectious in etiology. Furthermore, an atypical presentation of an oral infection may be the first sign of compromised immune function from an occult primary cancer, or it may represent evidence of relapse of a treated cancer.
History and examination
The history must include the cancer diagnosis and status; the nature, duration, and dates of previous and ongoing anti-neoplastic treatments; past medical history; current medications; drug allergies; prior history of oral and non-oral infections; and history of the present illness, including symptoms, onset, duration, any tests already ordered, and treatments rendered. A thorough past dental history, including recent visits; treatment for pulpal disease, including endodontic therapy; and a history of pericoronitis, recent extractions, and treatment for periodontal disease help to determine a patient’s risk for bacterial infection.
The patient should be evaluated for fever, and the area in question must be carefully examined and pulp testing done as necessary. Dental radiographs must be taken if an odontogenic source is suspected. Other imaging modalities such as CT and MRI may be required to evaluate the extent of the lesion. Serology, cultures, smears, and especially biopsy are often necessary to identify the process and suspected pathogen.
Bacterial infections
Odontogenic infections
Odontogenic infections, either of pulpal or periodontal origin, are frequently encountered in cancer patients. Any patient who has spontaneous or provoked dental pain, swelling, or evidence of purulent discharge must be considered to have an odontogenic infection until proven otherwise. As the inflammatory response may be muted due to myelosuppression, the clinical signs of erythema, swelling, and purulence are highly variable, and the absence of these features is insufficient to rule out infection.
Infections related to caries and nonvital teeth
In the presence of dental pain, large restorations, prior endodontic therapy, gross clinical caries, radiographic evidence of restorations or caries near the pulp, periapical radiolucencies, trismus, and dysphagia should all heighten the suspicion of an odontogenic infection . It is not uncommon for a previously asymptomatic endodontically treated tooth with a radiographically adequate apical seal to become symptomatic during severe neutropenia ( Fig. 1 ). This is most likely explained by an acute exacerbation of a subclinical chronic periapical infection, rather than true acute failure of endodontic therapy.
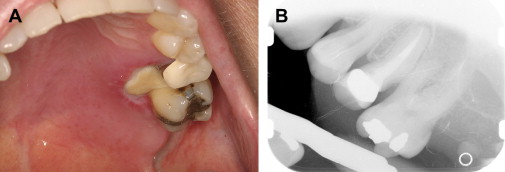
Periapical infections arising from posterior maxillary teeth may perforate the Schneidarian membrane and cause a sinusitis characterized by pain, headaches, and maxillary tenderness. Imaging with intraoral radiographs and maxillary sinus CT are required for diagnosis. Treatment includes eliminating the source of infection as well as at least 3 to 4 weeks of antimicrobial therapy directed at both oral flora and sinus pathogens . When multiple adjacent maxillary teeth present with pain, a primary bacterial or fungal acute sinusitis should be suspected.
Empiric therapy with clindamycin or amoxicillin/clavaunate should be initiated for periapic infections owing to potential penicillin resistance . Incision and drainage may be appropriate in the presence of a fluctuant swelling, or extractions may be indicated, first ensuring that there are adequate platelets . Although 50,000 platelets/μL is ideal, patients who have lower platelet levels may undergo tooth extraction if rigorous local methods to control bleeding are performed (see the article by Brennan and colleagues elsewhere in this issue). Patients may also require antibacterials and chlorhexidine oral rinses and should be followed up until the areas are healed.
Progression of periapic infections that are untreated or unresponsive to treatment may lead to osteomyelitis of the jaws, resulting in swelling, pain, suppuration, sinus tract formation, bone sequestration, and a radiographically characteristic “moth-eaten” appearance . Oro-cutaneous fistulae may form. Patients who have metastatic bone disease treated with high-potency intravenous bisphosphonate therapy may develop a secondary osteomyelitis within areas of bisphosphonate-associated osteonecrosis of the jaw (see the article by Ruggerio and Woo elsewhere in this issue).
Treatment typically requires long-term broad-spectrum antibiotic therapy in both situations as the infections often contain polymicrobial oral flora; adequate surgical debridement and possibly hyperbaric oxygen therapy should be considered . In rare cases, localized spread of infection can result in cavernous sinus thrombosis or Ludwig angina. Each requires immediate hospitalization and aggressive treatment with intravenous antibiotic therapy and possible surgical management because of the high risk of mortality .
Periodontal infections and pericoronitis
Chronic periodontal disease of varying severity is ubiquitous in the adult population. Exacerbation of chronic periodontal disease may not exhibit the traditional clinical signs of inflammation and swelling in patients who have severe neutropenia . Although oral mucositis and neutropenia are well-established risk factors for Viridans streptococci bacteremia, marginal ulceration and infection of the periodontium also may lead to bacteremia, and the severity of periodontal disease and inflammation is also thought to be related to the risk of bacteremia . Rarely, periodontal infections may be one of the initial presenting signs of underlying malignancy ( Fig. 2 ).
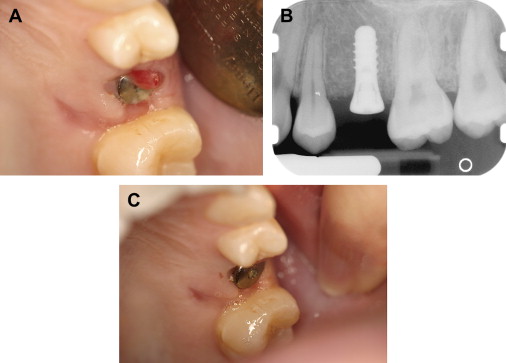
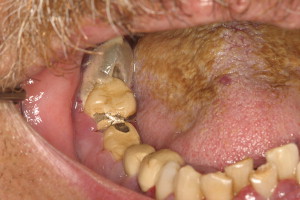
The evaluation should include examination for supra- and subgingival plaque and calculus, percussion, assessment of mobility, periodontal probing, and radiographic evaluation for bone loss and periodontal abscess formation . The course of periodontal destruction in chronically myelosuppressed individuals resembles what is often seen in patients who have poorly controlled diabetes mellitus in terms of both aggressive behavior and unpredictable response to conventional therapies, sometimes necessitating extraction of the involved teeth . Pericoronitis associated with partially erupted third molars often features ulceration and necrosis, which may represent an additional pathway for bacteremia ( Fig. 3 ) . Operculum removal or extraction may be indicated in severe cases or when profound extended neutropenia is anticipated (eg, allogeneic stem cell transplantation). Necrotizing ulcerative gingivitis and periodontitis present as painful ulcerations of the gingiva, sometimes with exposure of bone usually arising in areas of pre-existing periodontal infection; it may spread to the nonkeratinized tissues ( Fig. 4 ) .
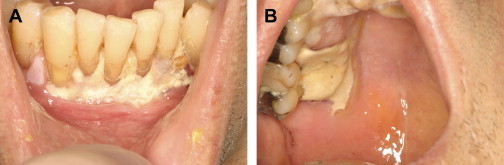
Treatment of acute exacerbation of periodontal disease includes scaling and curettage, extraction of hopeless teeth, and topical and systemic antimicrobial therapy, usually with agents that target oral anaerobic bacteria such as penicillin, clindamycin, or metronidazole.
Parotitis
Acute parotitis is an uncommon infectious complication of the parotid glands, characterized by a unilateral painful, indurated, erythematous swelling of the affected side of the face . This is usually caused by an ascending bacterial sialadenitis (most commonly Staphylococcus aureus ), and suppuration may be noted from the duct orifice. The principal risk factors in cancer patients are myelosuppression and decreased salivary flow, either due to chemotherapy, radiation, and/or dehydration. An MRI or a CT scan may be helpful in ruling out a neoplastic process or identifying a sialolith, respectively.
The empiric antibiotic of choice is amoxicillin/clavaunate or clindamycin therapy, but any purulent material should be sent for culture and antimicrobial susceptibility testing. Warm compresses to the site, adequate rehydration, and nutritional support are important adjunctive measures. Although exceedingly rare, infections such as tuberculous parotitis should be considered when there is parotid gland enlargement in chronically immunosuppressed patients .
Bacteremia
Gram-positive cocci, including Streptococcus viridans , have increasingly emerged as important agents of bacteremia in neutropenic patients . The presence of oral mucositis likely increases this risk as the mucosal barrier is breached; in patients being treated with hematopoietic cell transplantation (HCT), the severity of oral mucositis was found to be an independent predictor of anaerobic bacteremia . Cytopenic fevers are the most common clinical characteristic of bacteremia and may persist despite negative blood cultures . S mitis septicemia has been associated with a toxic shock-like syndrome characterized by hypotension, rash, palmar desquamation, and acute respiratory distress syndrome . Because of potential penicillin and cephalosporin resistance, vancomycin is used empirically to manage gram-positive cocci bacteremia in neutropenic cancer patients until the organism is identified and susceptibilities known .
Oral prophylactic antimicrobial therapy is considered in some centers in cancer patients during neutropenia, as some reports suggest this may decrease the incidence of bacteremia; however, the potential toxicity and emergence of resistant organisms must be carefully considered . Prophylaxis with quinolones is often used in this setting owing to their broad-spectrum activity, lack of myelosuppression, and good tolerability . Other ways to reduce bacteremia include use of granulocyte-colony-stimulating factor to reduce the period of profound neutropenia and measures to reduce oral mucositis such as palifermin .
Bacterial infections
Odontogenic infections
Odontogenic infections, either of pulpal or periodontal origin, are frequently encountered in cancer patients. Any patient who has spontaneous or provoked dental pain, swelling, or evidence of purulent discharge must be considered to have an odontogenic infection until proven otherwise. As the inflammatory response may be muted due to myelosuppression, the clinical signs of erythema, swelling, and purulence are highly variable, and the absence of these features is insufficient to rule out infection.
Infections related to caries and nonvital teeth
In the presence of dental pain, large restorations, prior endodontic therapy, gross clinical caries, radiographic evidence of restorations or caries near the pulp, periapical radiolucencies, trismus, and dysphagia should all heighten the suspicion of an odontogenic infection . It is not uncommon for a previously asymptomatic endodontically treated tooth with a radiographically adequate apical seal to become symptomatic during severe neutropenia ( Fig. 1 ). This is most likely explained by an acute exacerbation of a subclinical chronic periapical infection, rather than true acute failure of endodontic therapy.
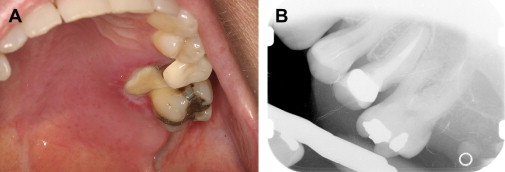
Periapical infections arising from posterior maxillary teeth may perforate the Schneidarian membrane and cause a sinusitis characterized by pain, headaches, and maxillary tenderness. Imaging with intraoral radiographs and maxillary sinus CT are required for diagnosis. Treatment includes eliminating the source of infection as well as at least 3 to 4 weeks of antimicrobial therapy directed at both oral flora and sinus pathogens . When multiple adjacent maxillary teeth present with pain, a primary bacterial or fungal acute sinusitis should be suspected.
Empiric therapy with clindamycin or amoxicillin/clavaunate should be initiated for periapic infections owing to potential penicillin resistance . Incision and drainage may be appropriate in the presence of a fluctuant swelling, or extractions may be indicated, first ensuring that there are adequate platelets . Although 50,000 platelets/μL is ideal, patients who have lower platelet levels may undergo tooth extraction if rigorous local methods to control bleeding are performed (see the article by Brennan and colleagues elsewhere in this issue). Patients may also require antibacterials and chlorhexidine oral rinses and should be followed up until the areas are healed.
Progression of periapic infections that are untreated or unresponsive to treatment may lead to osteomyelitis of the jaws, resulting in swelling, pain, suppuration, sinus tract formation, bone sequestration, and a radiographically characteristic “moth-eaten” appearance . Oro-cutaneous fistulae may form. Patients who have metastatic bone disease treated with high-potency intravenous bisphosphonate therapy may develop a secondary osteomyelitis within areas of bisphosphonate-associated osteonecrosis of the jaw (see the article by Ruggerio and Woo elsewhere in this issue).
Treatment typically requires long-term broad-spectrum antibiotic therapy in both situations as the infections often contain polymicrobial oral flora; adequate surgical debridement and possibly hyperbaric oxygen therapy should be considered . In rare cases, localized spread of infection can result in cavernous sinus thrombosis or Ludwig angina. Each requires immediate hospitalization and aggressive treatment with intravenous antibiotic therapy and possible surgical management because of the high risk of mortality .
Periodontal infections and pericoronitis
Chronic periodontal disease of varying severity is ubiquitous in the adult population. Exacerbation of chronic periodontal disease may not exhibit the traditional clinical signs of inflammation and swelling in patients who have severe neutropenia . Although oral mucositis and neutropenia are well-established risk factors for Viridans streptococci bacteremia, marginal ulceration and infection of the periodontium also may lead to bacteremia, and the severity of periodontal disease and inflammation is also thought to be related to the risk of bacteremia . Rarely, periodontal infections may be one of the initial presenting signs of underlying malignancy ( Fig. 2 ).
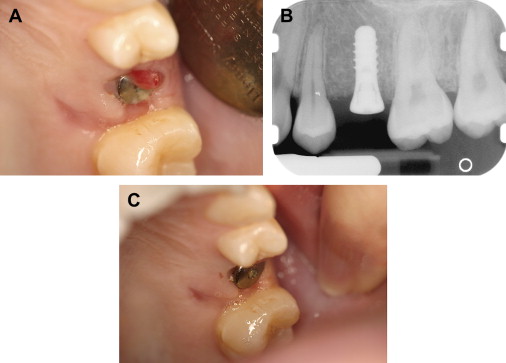
The evaluation should include examination for supra- and subgingival plaque and calculus, percussion, assessment of mobility, periodontal probing, and radiographic evaluation for bone loss and periodontal abscess formation . The course of periodontal destruction in chronically myelosuppressed individuals resembles what is often seen in patients who have poorly controlled diabetes mellitus in terms of both aggressive behavior and unpredictable response to conventional therapies, sometimes necessitating extraction of the involved teeth . Pericoronitis associated with partially erupted third molars often features ulceration and necrosis, which may represent an additional pathway for bacteremia ( Fig. 3 ) . Operculum removal or extraction may be indicated in severe cases or when profound extended neutropenia is anticipated (eg, allogeneic stem cell transplantation). Necrotizing ulcerative gingivitis and periodontitis present as painful ulcerations of the gingiva, sometimes with exposure of bone usually arising in areas of pre-existing periodontal infection; it may spread to the nonkeratinized tissues ( Fig. 4 ) .
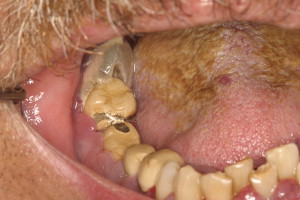
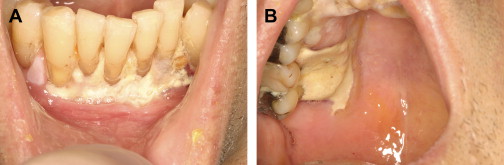
Treatment of acute exacerbation of periodontal disease includes scaling and curettage, extraction of hopeless teeth, and topical and systemic antimicrobial therapy, usually with agents that target oral anaerobic bacteria such as penicillin, clindamycin, or metronidazole.
Parotitis
Acute parotitis is an uncommon infectious complication of the parotid glands, characterized by a unilateral painful, indurated, erythematous swelling of the affected side of the face . This is usually caused by an ascending bacterial sialadenitis (most commonly Staphylococcus aureus ), and suppuration may be noted from the duct orifice. The principal risk factors in cancer patients are myelosuppression and decreased salivary flow, either due to chemotherapy, radiation, and/or dehydration. An MRI or a CT scan may be helpful in ruling out a neoplastic process or identifying a sialolith, respectively.
The empiric antibiotic of choice is amoxicillin/clavaunate or clindamycin therapy, but any purulent material should be sent for culture and antimicrobial susceptibility testing. Warm compresses to the site, adequate rehydration, and nutritional support are important adjunctive measures. Although exceedingly rare, infections such as tuberculous parotitis should be considered when there is parotid gland enlargement in chronically immunosuppressed patients .
Bacteremia
Gram-positive cocci, including Streptococcus viridans , have increasingly emerged as important agents of bacteremia in neutropenic patients . The presence of oral mucositis likely increases this risk as the mucosal barrier is breached; in patients being treated with hematopoietic cell transplantation (HCT), the severity of oral mucositis was found to be an independent predictor of anaerobic bacteremia . Cytopenic fevers are the most common clinical characteristic of bacteremia and may persist despite negative blood cultures . S mitis septicemia has been associated with a toxic shock-like syndrome characterized by hypotension, rash, palmar desquamation, and acute respiratory distress syndrome . Because of potential penicillin and cephalosporin resistance, vancomycin is used empirically to manage gram-positive cocci bacteremia in neutropenic cancer patients until the organism is identified and susceptibilities known .
Oral prophylactic antimicrobial therapy is considered in some centers in cancer patients during neutropenia, as some reports suggest this may decrease the incidence of bacteremia; however, the potential toxicity and emergence of resistant organisms must be carefully considered . Prophylaxis with quinolones is often used in this setting owing to their broad-spectrum activity, lack of myelosuppression, and good tolerability . Other ways to reduce bacteremia include use of granulocyte-colony-stimulating factor to reduce the period of profound neutropenia and measures to reduce oral mucositis such as palifermin .
Fungal infections
Oropharyngeal candidiasis (OPC) is the most common fungal infection in cancer patients, and Candida albicans , a commensal organism found in 34% to 68% of the normal healthy population, is the most common causative agent . The incidence of OPC in cancer patients is variable and is influenced by the underlying disease, immune status, intensity of anti-neoplastic treatment, and salivary gland function . For example, before treatment, 50% of head and neck cancer patients have oral Candida colonization; however, this increases to nearly 70% during active radiotherapy . In the United States, the estimated incidence of disseminated candidiasis is 60,000 to 70,000 cases per year and is associated with health care costs of $2 to 4 billion per year .
Deep fungal infections must be considered in patients who have nonhealing ulcerated lesions . Both environmental opportunistic molds, such as Aspergillus and Zygomyces , and endemic fungi, such as Histoplasma capsulatum , should be considered. It is important to carefully assess exposures such as hobbies and social activities (eg, gardening, spelunking, and use of marijuana) and places of previous residence to properly assess potential exposures. When deep fungal infections are suspected, clinically early pathogen-specific antigen testing (eg, histoplasma antigen and A galactomannan antibody) and biopsy are essential for diagnosis. Imaging studies, including CT and/or MRI, may be indicated to rule out deeper processes such as sinus, osseous, and fascial/soft-tissue involvement and to evaluate for disseminated disease .
Clinical presentation
Pseudomembranous candidiasis (“thrush”), erythematous candidiasis, hyperplastic candidiasis, and angular cheilitis are all forms of OPC, whose symptoms may include generalized discomfort, dysgeusia, xerostomia, and burning. Pseudomembranous candidiasis is the most common form and presents as symptomatic or asymptomatic white papules or plaques that can be wiped away with gauze, typically revealing a raw and erythematous base ( Fig. 5 ). More commonly encountered in patients who have profound salivary gland hypofunction, erythematous candidiasis presents as an atrophic red lesion that is often associated with burning symptoms. The tongue and palate are typically affected, the former appearing smooth and depapillated. Rarely, OPC presents as a distinct white plaque (hyperplastic candidiasis) that cannot be rubbed away and may require biopsy to distinguish this lesion from other white lesions. Although any oral mucosal surface may be involved, the palate, oropharynx, and tongue are most commonly affected. Patients who have angular cheilitis present with painful, dry, and cracked corners of the mouth that may easily bleed. This is frequently seen in combination with one of the intraoral forms of infection.
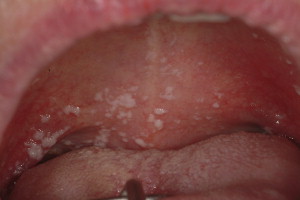
Risk factors
There are a number of factors that increase the cancer patient’s risk of developing OPC ( Box 1 ). Salivary gland hypofunction is one of the most common causes of recurrent OPC. Saliva possesses critical antimicrobial, fungistatic, and/or fungicidal protective elements that include histatins, secretory IgA, secretory component, lysozyme, mucins, statherin, transferrin, lactoferrin, and defensins . These proteins and peptides inhibit adhesion and multiplication of Candida on mucosal surfaces . Saliva also contains phagocytes that help to regulate the growth of Candida . When saliva is reduced, there is a loss of these innate protective mechanisms, therefore setting the stage for the development of OPC. Radiotherapy to the head and neck results in irreversible salivary gland damage with a decrease in saliva production after as little as 20 Gy and increased viscosity . Chemotherapy may cause reversible salivary gland hypofunction . Many patients are also taking medications that have anticholinergic leading to salivary gland hypofunction. Antibiotics and corticosteroid use also increase the risk for candidiasis .
- •
Neutropenia (severity, duration)
- •
Use of broad-spectrum antibiotics
- •
Use of corticosteroids
- •
Salivary gland hypofunction
- •
Total body irradiation
- •
Colonization of multiple body sites
- •
Increased length of hospital stay
- •
Wearing of dentures
Diagnosis
Diagnosis can often be made by clinical inspection alone, by cytologic smear, or in some cases may require speciation by culture. Classic pseudomembranous candidiasis should be treated empirically and does not typically require culture. However, in patients undergoing chemo- or radiotherapy, what appears to be erythematous candidiasis may well be the early signs of treatment-related mucosal toxicity . When lesions are nonresponsive to therapeutic antifungal therapy, sensitivity studies should be considered. Biopsy of persistent white, erythematous, or in particular, solitary nonhealing ulcerated lesions is indicated to rule out an underlying deep fungal infection.
Topical management
The Infectious Diseases Society of America (IDSA) has published guidelines for the treatment of OPC in cancer patients . Initial episodes of OPC can be treated with clotrimazole (an azole) troches or nystatin (a polyene) oral suspension or pastilles for 7 to14 days ( Table 1 ). Both compounds bind to the fungal/yeast cell membrane and alter permeability, resulting in the loss of essential intracellular elements . Clotrimazole is a moderate inhibitor of cytochrome P450 3A4 and could result in elevated levels of several medications, including tacrolimus, cyclosporine, benzodiazepines, and HMG-Co A reductase inhibitors . Clotrimazole and nystatin oral formulations contain dextrose and sucrose, respectively, and can increase a patient’s risk for the development of dental caries as well as affect blood glucose levels in diabetics. Amphotericin B (AmB) is another polyene antifungal agent that has been used infrequently in topical formulations (eg, lozenges, suspensions) for the treatment of refractory OPC . Oral appliances/dentures may act as fomites and should be disinfected by soaking in nystatin, chlorhexidine gluconate oral rinse, dilute sodium hypochlorite (1:10), or sodium benzoate 20 to 30 minutes daily. Appliances should also be left out of the mouth during sleep.
Systemic management
When systemic therapy is required, the ISDA guidelines recommend the use of first-generation triazoles (fluconazole or itraconazole, 100–200 mg/day) for 7 to 14 days for the treatment of OPC. Itraconazole inhibits fungal cell membrane formation and interferes with cytochrome P 450 activity. There have been rare serious cardiovascular events associated with itraconazole, and its use is contraindicated if a patient is taking cisapride, pimozide, and quinidine . Voriconazole and posaconazole are newer triazoles that may be effective against fluconazole- and itraconazole-resistant strains of Candida and other non- albicans species , although success rates in this setting are approximately 70% owing to cross-azole resistance.
Echinocandins (caspofungin, micafungin, anidulafungin) and AmB formulations (AmB deoxycholate, lipid-based formulations) are systemic agents that may be used in refractory cases of clinically severe OPC. The antifungal spectrum of caspofungin is relatively narrow and includes Aspergillus and Candida spp , whereas AmB has a broader spectrum of activity. A recent Cochrane review of treatment interventions specifically for OPC in cancer patients concludes that there is weak and unreliable evidence to support which agents should be prescribed . In the present authors’ experience, topical antifungal treatment is sufficient for most cases of mild to moderate OPC. For patients who have symptomatic disease whose current immune status makes them likely to relapse, fluconazole is an effective option for treatment and suppressive therapy until chemotherapy is completed or immunosuppression lowered. Use of intravenous agents, voriconazole, or posaconazole should be reserved for patients who have proven OPC that is drug resistant or for those who are intolerant to fluconazole.
Prevention
In cancer and immunosuppressed patients, the goal of antifungal prophylaxis is to avoid the increased morbidity/mortality associated with the development of OPC. The choice of prophylactic drug regimen depends on the underlying malignancy, expected course of neutropenia, and/or expected severity/extent of oropharyngeal mucositis . Drug toxicity, financial costs, and resistance are important considerations in the decision to implement antifungal prophylaxis .
Chlorhexidine inhibits fungal cell adherence to denture materials and epithelial cells and may be used for prophylaxis. However, nystatin and chlorhexidine in combination may reduce the antifungal properties of both medications . Prophylactic clotrimazole (10 mg, 5 times/day) has been evaluated in the advanced HIV-infected population and was found to be less effective than fluconazole tablets in the prevention of Candida -associated diseases . In patients undergoing HCT, topical antifungals have not been shown to reduce the incidence of invasive candidiasis .
The IDSA guidelines recommend fluconazole prophylaxis for patients undergoing HCT with anticipated prolonged neutropenia, mucosal damage, or who have recently received fludarabine or 2-chlorodeoxyadenosine . Fluconazole should be the choice in patients who have fluconazole-susceptible Candida and in health centers where C albicans is the predominant etiology of invasive fungal disease . In this setting, fluconazole (400 mg/day) is typically given during the neutropenic period (ANC <500 cells/mm 3 ) . This practice is controversial and should be guided by the incidence of fungal infections in the high-risk population. At transplantation centers where the incidence of fungal infections during the neutropenic period is low, fluconazole prophylaxis is not used. Alternatives to fluconazole for antifungal prophylaxis in patients undergoing cancer therapy (ie, chemotherapy and/or radiotherapy) include medications such as caspofungin or micafungin .
Resistance
Resistant strains of Candida spp, especially to fluconazole, are a concern in patients who are immunosuppressed, have had previous OPC, and/or require suppressive antifungal therapy . In addition, various studies have shown that the Candida spp often change throughout the course of and after radiotherapy/chemotherapy from mostly albicans to non- albicans species ; There are also differences in colonization based on whether or not a patient is ambulatory or hospitalized, and the stage of his or her cancer . Non- albicans spp that have been identified in cancer patients include C glabrata , C krusei , C tropicalis , and C dubliniensis ; they are often resistant to conventional antifungal agents. When compared with C albicans, C glabrata exhibits varying degrees of resistance to the antifungal activity of some salivary proteins and to antifungal medications . Ueta and colleagues showed in vitro that anticancer medications and radiotherapy result in increased Candida proliferation, adherence, and antifungal drug resistance. In breakthrough OPC, culture and sensitivity testing can help with choosing an alternative antifungal drug. These tests can better elucidate if the infection is due to a resistant strain and/or to different species with varying sensitivities. As the clinical and laboratory markers of Candidia colonization/active infection improve with advances in diagnostic technology, there is hope that antifungal therapeutics can be tailored to a patient’s colonization profile .
Management of deep fungal infections
Management of invasive mold infection requires a multi-tiered approach that includes (1) minimizing immunosuppression (eg, deceasing corticosteroid dose and facilitating neutrophil recovery); (2) assessing for and controlling permissive viral infections (eg, cytomegalovirus infection); (3) adequate surgical debridement, especially for the vasotrophic molds (eg, Zygomyces and Aspergillus ); and (4) targeted antifungal therapy. Depending on the severity of illness, degree of organ insufficiency (eg, renal or hepatic dysfunction), and the organism causing invasive disease, a polyene (eg, amphotericin B product), an azole (eg, voriconazole or posaconazole), or an echinocandin (eg, caspofungin, micafungin, or anidulafungin) could be used .
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


