The success of endodontic treatment depends on the eradication of microbes from the root-canal system and prevention of reinfection. The root canal is shaped with hand and rotary instruments under constant irrigation to remove the inflamed and necrotic tissue, microbes/biofilms, and other debris from the root-canal space. Irrigants have traditionally been delivered into the root-canal space using syringes and metal needles of different size and tip design. Clinical experience and research have shown, however, that this classic approach typically results in ineffective irrigation. Many of the compounds used for irrigation have been chemically modified and several mechanical devices have been developed to improve the penetration and effectiveness of irrigation. This article summarizes the chemistry, biology, and procedures for safe and efficient irrigation and provides cutting-edge information on the most recent developments.
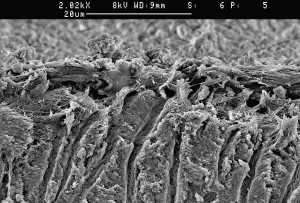
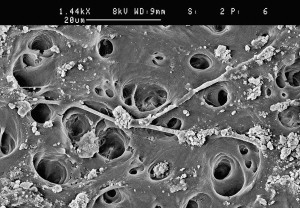
The success of endodontic treatment depends on the eradication of microbes (if present) from the root-canal system and prevention of reinfection. The root canal is shaped with hand and rotary instruments under constant irrigation to remove the inflamed and necrotic tissue, microbes/biofilms, and other debris from the root-canal space. The main goal of instrumentation is to facilitate effective irrigation, disinfection, and filling. Several studies using advanced techniques such as microcomputed tomography (CT) scanning have demonstrated that proportionally large areas of the main root-canal wall remain untouched by the instruments, emphasizing the importance of chemical means of cleaning and disinfecting all areas of the root canal ( Figs. 1 and 2 ). There is no single irrigating solution that alone sufficiently covers all of the functions required from an irrigant. Optimal irrigation is based on the combined use of 2 or several irrigating solutions, in a specific sequence, to predictably obtain the goals of safe and effective irrigation. Irrigants have traditionally been delivered into the root-canal space using syringes and metal needles of different size and tip design. Clinical experience and research have shown, however, that this classic approach typically results in ineffective irrigation, particularly in peripheral areas such as anastomoses between canals, fins, and the most apical part of the main root canal. Therefore, many of the compounds used for irrigation have been chemically modified and several mechanical devices have been developed to improve the penetration and effectiveness of irrigation. This article summarizes the chemistry, biology, and procedures for safe and efficient irrigation and provides cutting-edge information on the most recent developments.
Goals of irrigation
Irrigation has a central role in endodontic treatment. During and after instrumentation, the irrigants facilitate removal of microorganisms, tissue remnants, and dentin chips from the root canal through a flushing mechanism ( Box 1 ). Irrigants can also help prevent packing of the hard and soft tissue in the apical root canal and extrusion of infected material into the periapical area. Some irrigating solutions dissolve either organic or inorganic tissue in the root canal. In addition, several irrigating solutions have antimicrobial activity and actively kill bacteria and yeasts when introduced in direct contact with the microorganisms. However, several irrigating solutions also have cytotoxic potential, and they may cause severe pain if they gain access into the periapical tissues. An optimal irrigant should have all or most of the positive characteristics listed in Box 1 , but none of the negative or harmful properties. None of the available irrigating solutions can be regarded as optimal. Using a combination of products in the correct irrigation sequence contributes to a successful treatment outcome.
- •
Washing action (helps remove debris)
- •
Reduce instrument friction during preparation (lubricant)
- •
Facilitate dentin removal (lubricant)
- •
Dissolve inorganic tissue (dentin)
- •
Penetrate to canal periphery
- •
Dissolve organic matter (dentin collagen, pulp tissue, biofilm)
- •
Kill bacteria and yeasts (also in biofilm)
- •
Do not irritate or damage vital periapical tissue, no caustic or cytotoxic effects
- •
Do not weaken tooth structure
Irrigating solutions
Sodium Hypochlorite
Sodium hypochlorite (NaOCl) is the most popular irrigating solution. NaOCl ionizes in water into Na + and the hypochlorite ion, OCl − , establishing an equilibrium with hypochlorous acid (HOCl). At acidic and neutral pH, chlorine exists predominantly as HOCl, whereas at high pH of 9 and above, OCl − predominates. Hypochlorous acid is responsible for the antibacterial activity; the OCl − ion is less effective than the undissolved HOCl. Hypochloric acid disrupts several vital functions of the microbial cell, resulting in cell death.
NaOCl is commonly used in concentrations between 0.5% and 6%. It is a potent antimicrobial agent, killing most bacteria instantly on direct contact. It also effectively dissolves pulpal remnants and collagen, the main organic components of dentin. Hypochlorite is the only root-canal irrigant of those in general use that dissolves necrotic and vital organic tissue. It is difficult to imagine successful irrigation of the root canal without hypochlorite. Although hypochlorite alone does not remove the smear layer, it affects the organic part of the smear layer, making its complete removal possible by subsequent irrigation with EDTA or citric acid (CA). It is used as an unbuffered solution at pH 11 in the various concentrations mentioned earlier, or buffered with bicarbonate buffer (pH 9.0), usually as a 0.5% (Dakin solution) or 1% solution. However, buffering does not seem to have any major effect on the properties of NaOCl, contrary to earlier belief.
There is considerable variation in the literature regarding the antibacterial effect of NaOCl. In some articles hypochlorite is reported to kill the target microorganisms in seconds, even at low concentrations, although other reports have published considerably longer times for the killing of the same species. Such differences are a result of confounding factors in some of the studies. The presence of organic matter during the killing experiments has a great effect on the antibacterial activity of NaOCl. Haapasalo and colleagues showed that the presence of dentin caused marked delays in the killing of Enterococcus faecalis by 1% NaOCl. Many of the earlier studies were performed in the presence of an unknown amount of organic matter (eg, nutrient broth) or without controlling the pH of the culture, both of which affect the result. When the confounding factors are eliminated, it has been shown that NaOCl kills the target microorganisms rapidly even at low concentrations of less than 0.1%. However, in vivo the presence of organic matter (inflammatory exudate, tissue remnants, microbial biomass) consumes NaOCl and weakens its effect. Therefore, continuous irrigation and time are important factors for the effectiveness of hypochlorite.
Byström and Sundqvist studied the irrigation of root canals that were necrotic and contained a mixture of anaerobic bacteria. These investigators showed that using 0.5% or 5% NaOCl, with or without EDTA for irrigation, resulted in considerable reduction of bacterial counts in the canal when compared with irrigation with saline. However, it was difficult to render the canals completely free from bacteria, even after repeated sessions. Siqueira and colleagues reported similar results using root canals infected with E faecalis . Both studies failed to show a significant difference in the antibacterial efficacy between the low and high concentrations of NaOCl. Contrary to these results, Clegg and colleagues, in an ex vivo biofilm study, demonstrated a strong difference in the effectiveness against biofilm bacteria by 6% and 3% NaOCl, the higher concentration being more effective.
The weaknesses of NaOCl include the unpleasant taste, toxicity, and its inability to remove the smear layer ( Fig. 3 ) by itself, as it dissolves only organic material. The limited antimicrobial effectiveness of NaOCl in vivo is also disappointing. The poorer in vivo performance compared with in vitro is probably caused by problems in penetration to the most peripheral parts of the root-canal system such as fins, anastomoses, apical canal, lateral canals, and dentin canals. Also, the presence of inactivating substances such as exudate from the periapical area, pulp tissue, dentin collagen, and microbial biomass counteract the effectiveness of NaOCl. Recently, it has been shown by in vitro studies that long-term exposure of dentin to a high concentration sodium hypochlorite can have a detrimental effect on dentin elasticity and flexural strength. Although there are no clinical data on this phenomenon, it raises the question of whether hypochlorite in some situations may increase the risk of vertical root fracture.

In summary, sodium hypochlorite is the most important irrigating solution and the only one capable of dissolving organic tissue, including biofilm and the organic part of the smear layer. It should be used throughout the instrumentation phase. However, use of hypochlorite as the final rinse following EDTA or CA rapidly produces severe erosion of the canal-wall dentin and should probably be avoided.
EDTA and CA
Complete cleaning of the root-canal system requires the use of irrigants that dissolve organic and inorganic material. As hypochlorite is active only against the former, other substances must be used to complete the removal of the smear layer and dentin debris. EDTA and CA effectively dissolve inorganic material, including hydroxyapatite. They have little or no effect on organic tissue and alone they do not have antibacterial activity, despite some conflicting reports on EDTA. EDTA is most commonly used as a 17% neutralized solution (disodium EDTA, pH 7), but a few reports have indicated that solutions with lower concentrations (eg, 10%, 5%, and even 1%) remove the smear layer equally well after NaOCl irrigation. Considering the high cost of EDTA, it may be worthwhile to consider using diluted EDTA. CA is also marketed and used in various concentrations, ranging from 1% to 50%, with a 10% solution being the most common. EDTA and CA are used for 2 to 3 minutes at the end of instrumentation and after NaOCl irrigation. Removal of the smear layer by EDTA or CA improves the antibacterial effect of locally used disinfecting agents in deeper layers of dentin. EDTA and CA are manufactured as liquids and gels. Although there are no comparative studies about the effectiveness of liquid and gel products to demineralize dentin, it is possible that the small volume of the root canal (only a few microliters) contributes to a rapid saturation of the chemical and thereby loss of effectiveness. In such situations, the use of liquid products and continuous irrigation should be recommended.
Chlorhexidine Digluconate
Chlorhexidine digluconate (CHX) is widely used in disinfection in dentistry because of its good antimicrobial activity. It has gained considerable popularity in endodontics as an irrigating solution and as an intracanal medicament. CHX does not possess some of the undesired characteristics of sodium hypochlorite (ie, bad smell and strong irritation to periapical tissues). However, CHX has no tissue-dissolving capability and therefore it cannot replace sodium hypochlorite.
CHX permeates the microbial cell wall or outer membrane and attacks the bacterial cytoplasmic or inner membrane or the yeast plasma membrane. In high concentrations, CHX causes coagulation of intracellular components. One of the reasons for the popularity of CHX is its substantivity (ie, continued antimicrobial effect), because CHX binds to hard tissue and remains antimicrobial. However, similar to other endodontic disinfecting agents, the activity of CHX depends on the pH and is also greatly reduced in the presence of organic matter.
Several studies have compared the antibacterial effect of NaOCl and 2% CHX against intracanal infection and have shown little or no difference between their antimicrobial effectiveness. Although bacteria may be killed by CHX, the biofilm and other organic debris are not removed by it. Residual organic tissue may have a negative effect on the quality of the seal by the permanent root filling, necessitating the use of NaOCl during instrumentation. However, CHX does not cause erosion of dentin like NaOCl does as the final rinse after EDTA, and therefore 2% CHX may be a good choice for maximized antibacterial effect at the end of the chemomechanical preparation.
Most of the research on the use of CHX in endodontics is carried out using in vitro and ex vivo models and gram-positive test organisms, mostly E faecalis . It is therefore possible that the studies have given an overpositive picture of the usefulness of CHX as an antimicrobial agent in endodontics. More research is needed to identify the optimal irrigation regimen for various types of endodontic treatments. CHX is marketed as a water-based solution and as a gel (with Natrosol). Some studies have indicated that the CHX gel has a slightly better performance than the CHX liquid but the reasons for possible differences are not known.
Other Irrigating Solutions
Other irrigating solutions used in endodontics have included sterile water, physiologic saline, hydrogen peroxide, urea peroxide, and iodine compounds. All of these except iodine compounds lack antibacterial activity when used alone, and they do not dissolve tissue either. Therefore there is no good reason for their use in canal irrigation in routine cases. In addition, water and saline solutions bear the risk of contamination if used from containers that have been opened more than once. Iodine potassium iodide (eg, 2% and 4%, respectively) has considerable antimicrobial activity but no tissue-dissolving capability and it could be used at the end of the chemomechanical preparation like CHX. However, some patients are allergic to iodine, which must be taken into consideration.
Interactions Between Irrigating Solutions
Hypochlorite and EDTA are the 2 most commonly used irrigating solutions. As they have different characteristics and tasks, it has been tempting to use them as a mixture. However, EDTA (and CA) instantaneously reduces the amount of chlorine when mixed with sodium hypochlorite, resulting in the loss of NaOCl activity. Thus, these solutions should not be mixed.
CHX has no tissue-dissolving activity and there have been efforts to combine CHX with hypochlorite for added benefits from the 2 solutions. However, CHX and NaOCl are not soluble in each other; a brownish-orange precipitate is formed when they are mixed ( Fig. 4 ). The characteristics of the precipitate and the liquid phase have not been thoroughly examined, but the precipitate prevents the clinical use of the mixture. Atomic absorption spectrophotometry has indicated that the precipitate contains iron, which may be the reason for the orange development. Presence of parachloroaniline, which may have mutagenic potential, has also been demonstrated in the precipitate.

Mixing CHX and EDTA immediately produces a white precipitate ( Fig. 5 ). Although the properties of the mixture and the cleared supernatant have not been thoroughly studied, it seems that the ability of EDTA to remove the smear layer is reduced.

Many clinicians mix NaOCl with hydrogen peroxide for root-canal irrigation. Despite more vigorous bubbling, the effectiveness of the mixture has not been shown to be better than that of NaOCl alone. However, combining hydrogen peroxide with CHX in an ex vivo model resulted in a considerable increase in the antibacterial activity of the mixture compared with the components alone in an infected dentin block. However, there are no data concerning the use or effectiveness of the mixture in clinical use.
Combination Products
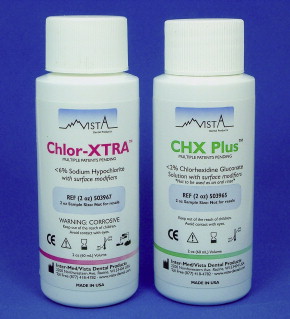
Although some of the main irrigating solutions cannot be mixed without loss of activity or development of potentially toxic by-products, several combination products are on the market, many with some evidence of improved activity and function. Surface-active agents have been added to several different types of irrigants to lower their surface tension and to improve their penetration in the root canal. In the hope of better smear-layer removal, detergents have been added to some EDTA preparations (eg, SmearClear ( Fig. 6 )) and hypoclorite (eg, Chlor-XTRA ( Fig. 7 ) and White King). Detergent addition has been shown to increase the speed of tissue dissolution by hypochlorite. No data are available on whether dentin penetration is also improved. Recently, a few studies have been published in which the antibacterial activity of a chlorhexidine product with surface-active agents (CHX-Plus; see Fig. 7 ) has been compared with regular CHX, both with 2% chlorhexidine concentrations. The studies have shown superior killing of planktonic and biofilm bacteria by the combination product. There are no studies about whether adding surface-active agents increases the risk of the irrigants escaping to the periapical area in clinical use.

MTAD (a mixture of tetracycline isomer, acid, and detergent, Biopure, Tulsa Dentsply, Tulsa, OK, USA) and Tetraclean are new combination products for root-canal irrigation that contain an antibiotic, doxycycline. MTAD and Tetraclean are designed primarily for smear-layer removal with added antimicrobial activity. Both contain CA, doxycycline, and a detergent. They differ from each other in CA concentration and type of detergent included. They do not dissolve organic tissue and are intended for use at the end of chemomechanical preparation after sodium hypochlorite. Although earlier studies showed promising antibacterial effects by MTAD, recent studies have indicated that an NaOCl/EDTA combination is equally or more effective than NaOCl/MTAD. Comparative studies on MATD and Tetraclean have indicated better antibacterial effects by the latter. Although a mixture containing an antibiotic may have good short-term and long-term effects, concerns have been expressed regarding the use of tetracycline (doxycycline) because of possible resistance to the antibiotic and staining of the tooth hard tissue, which has been demonstrated by exposure to light in an in vitro expreriment. However, no report of in vivo staining has been published.
Irrigating solutions
Sodium Hypochlorite
Sodium hypochlorite (NaOCl) is the most popular irrigating solution. NaOCl ionizes in water into Na + and the hypochlorite ion, OCl − , establishing an equilibrium with hypochlorous acid (HOCl). At acidic and neutral pH, chlorine exists predominantly as HOCl, whereas at high pH of 9 and above, OCl − predominates. Hypochlorous acid is responsible for the antibacterial activity; the OCl − ion is less effective than the undissolved HOCl. Hypochloric acid disrupts several vital functions of the microbial cell, resulting in cell death.
NaOCl is commonly used in concentrations between 0.5% and 6%. It is a potent antimicrobial agent, killing most bacteria instantly on direct contact. It also effectively dissolves pulpal remnants and collagen, the main organic components of dentin. Hypochlorite is the only root-canal irrigant of those in general use that dissolves necrotic and vital organic tissue. It is difficult to imagine successful irrigation of the root canal without hypochlorite. Although hypochlorite alone does not remove the smear layer, it affects the organic part of the smear layer, making its complete removal possible by subsequent irrigation with EDTA or citric acid (CA). It is used as an unbuffered solution at pH 11 in the various concentrations mentioned earlier, or buffered with bicarbonate buffer (pH 9.0), usually as a 0.5% (Dakin solution) or 1% solution. However, buffering does not seem to have any major effect on the properties of NaOCl, contrary to earlier belief.
There is considerable variation in the literature regarding the antibacterial effect of NaOCl. In some articles hypochlorite is reported to kill the target microorganisms in seconds, even at low concentrations, although other reports have published considerably longer times for the killing of the same species. Such differences are a result of confounding factors in some of the studies. The presence of organic matter during the killing experiments has a great effect on the antibacterial activity of NaOCl. Haapasalo and colleagues showed that the presence of dentin caused marked delays in the killing of Enterococcus faecalis by 1% NaOCl. Many of the earlier studies were performed in the presence of an unknown amount of organic matter (eg, nutrient broth) or without controlling the pH of the culture, both of which affect the result. When the confounding factors are eliminated, it has been shown that NaOCl kills the target microorganisms rapidly even at low concentrations of less than 0.1%. However, in vivo the presence of organic matter (inflammatory exudate, tissue remnants, microbial biomass) consumes NaOCl and weakens its effect. Therefore, continuous irrigation and time are important factors for the effectiveness of hypochlorite.
Byström and Sundqvist studied the irrigation of root canals that were necrotic and contained a mixture of anaerobic bacteria. These investigators showed that using 0.5% or 5% NaOCl, with or without EDTA for irrigation, resulted in considerable reduction of bacterial counts in the canal when compared with irrigation with saline. However, it was difficult to render the canals completely free from bacteria, even after repeated sessions. Siqueira and colleagues reported similar results using root canals infected with E faecalis . Both studies failed to show a significant difference in the antibacterial efficacy between the low and high concentrations of NaOCl. Contrary to these results, Clegg and colleagues, in an ex vivo biofilm study, demonstrated a strong difference in the effectiveness against biofilm bacteria by 6% and 3% NaOCl, the higher concentration being more effective.
The weaknesses of NaOCl include the unpleasant taste, toxicity, and its inability to remove the smear layer ( Fig. 3 ) by itself, as it dissolves only organic material. The limited antimicrobial effectiveness of NaOCl in vivo is also disappointing. The poorer in vivo performance compared with in vitro is probably caused by problems in penetration to the most peripheral parts of the root-canal system such as fins, anastomoses, apical canal, lateral canals, and dentin canals. Also, the presence of inactivating substances such as exudate from the periapical area, pulp tissue, dentin collagen, and microbial biomass counteract the effectiveness of NaOCl. Recently, it has been shown by in vitro studies that long-term exposure of dentin to a high concentration sodium hypochlorite can have a detrimental effect on dentin elasticity and flexural strength. Although there are no clinical data on this phenomenon, it raises the question of whether hypochlorite in some situations may increase the risk of vertical root fracture.

In summary, sodium hypochlorite is the most important irrigating solution and the only one capable of dissolving organic tissue, including biofilm and the organic part of the smear layer. It should be used throughout the instrumentation phase. However, use of hypochlorite as the final rinse following EDTA or CA rapidly produces severe erosion of the canal-wall dentin and should probably be avoided.
EDTA and CA
Complete cleaning of the root-canal system requires the use of irrigants that dissolve organic and inorganic material. As hypochlorite is active only against the former, other substances must be used to complete the removal of the smear layer and dentin debris. EDTA and CA effectively dissolve inorganic material, including hydroxyapatite. They have little or no effect on organic tissue and alone they do not have antibacterial activity, despite some conflicting reports on EDTA. EDTA is most commonly used as a 17% neutralized solution (disodium EDTA, pH 7), but a few reports have indicated that solutions with lower concentrations (eg, 10%, 5%, and even 1%) remove the smear layer equally well after NaOCl irrigation. Considering the high cost of EDTA, it may be worthwhile to consider using diluted EDTA. CA is also marketed and used in various concentrations, ranging from 1% to 50%, with a 10% solution being the most common. EDTA and CA are used for 2 to 3 minutes at the end of instrumentation and after NaOCl irrigation. Removal of the smear layer by EDTA or CA improves the antibacterial effect of locally used disinfecting agents in deeper layers of dentin. EDTA and CA are manufactured as liquids and gels. Although there are no comparative studies about the effectiveness of liquid and gel products to demineralize dentin, it is possible that the small volume of the root canal (only a few microliters) contributes to a rapid saturation of the chemical and thereby loss of effectiveness. In such situations, the use of liquid products and continuous irrigation should be recommended.
Chlorhexidine Digluconate
Chlorhexidine digluconate (CHX) is widely used in disinfection in dentistry because of its good antimicrobial activity. It has gained considerable popularity in endodontics as an irrigating solution and as an intracanal medicament. CHX does not possess some of the undesired characteristics of sodium hypochlorite (ie, bad smell and strong irritation to periapical tissues). However, CHX has no tissue-dissolving capability and therefore it cannot replace sodium hypochlorite.
CHX permeates the microbial cell wall or outer membrane and attacks the bacterial cytoplasmic or inner membrane or the yeast plasma membrane. In high concentrations, CHX causes coagulation of intracellular components. One of the reasons for the popularity of CHX is its substantivity (ie, continued antimicrobial effect), because CHX binds to hard tissue and remains antimicrobial. However, similar to other endodontic disinfecting agents, the activity of CHX depends on the pH and is also greatly reduced in the presence of organic matter.
Several studies have compared the antibacterial effect of NaOCl and 2% CHX against intracanal infection and have shown little or no difference between their antimicrobial effectiveness. Although bacteria may be killed by CHX, the biofilm and other organic debris are not removed by it. Residual organic tissue may have a negative effect on the quality of the seal by the permanent root filling, necessitating the use of NaOCl during instrumentation. However, CHX does not cause erosion of dentin like NaOCl does as the final rinse after EDTA, and therefore 2% CHX may be a good choice for maximized antibacterial effect at the end of the chemomechanical preparation.
Most of the research on the use of CHX in endodontics is carried out using in vitro and ex vivo models and gram-positive test organisms, mostly E faecalis . It is therefore possible that the studies have given an overpositive picture of the usefulness of CHX as an antimicrobial agent in endodontics. More research is needed to identify the optimal irrigation regimen for various types of endodontic treatments. CHX is marketed as a water-based solution and as a gel (with Natrosol). Some studies have indicated that the CHX gel has a slightly better performance than the CHX liquid but the reasons for possible differences are not known.
Other Irrigating Solutions
Other irrigating solutions used in endodontics have included sterile water, physiologic saline, hydrogen peroxide, urea peroxide, and iodine compounds. All of these except iodine compounds lack antibacterial activity when used alone, and they do not dissolve tissue either. Therefore there is no good reason for their use in canal irrigation in routine cases. In addition, water and saline solutions bear the risk of contamination if used from containers that have been opened more than once. Iodine potassium iodide (eg, 2% and 4%, respectively) has considerable antimicrobial activity but no tissue-dissolving capability and it could be used at the end of the chemomechanical preparation like CHX. However, some patients are allergic to iodine, which must be taken into consideration.
Interactions Between Irrigating Solutions
Hypochlorite and EDTA are the 2 most commonly used irrigating solutions. As they have different characteristics and tasks, it has been tempting to use them as a mixture. However, EDTA (and CA) instantaneously reduces the amount of chlorine when mixed with sodium hypochlorite, resulting in the loss of NaOCl activity. Thus, these solutions should not be mixed.
CHX has no tissue-dissolving activity and there have been efforts to combine CHX with hypochlorite for added benefits from the 2 solutions. However, CHX and NaOCl are not soluble in each other; a brownish-orange precipitate is formed when they are mixed ( Fig. 4 ). The characteristics of the precipitate and the liquid phase have not been thoroughly examined, but the precipitate prevents the clinical use of the mixture. Atomic absorption spectrophotometry has indicated that the precipitate contains iron, which may be the reason for the orange development. Presence of parachloroaniline, which may have mutagenic potential, has also been demonstrated in the precipitate.





