If one driving force has driven and propagated cosmetic facial surgery over the past 20 years, it is facial fillers. Volume loss is one of the prime factors that cause an aged look. Of late, much focus has been placed on injectable fillers. Today’s cosmetic surgery patients are extremely savvy on the pros and cons of specific procedures. They want to look younger and not just tighter. The emphasis of past surgeons was to help patients look tighter, and little attention was paid to volume restoration. With the new era of cosmetic sophistication, fillers (and facial implants) may serve as the icing on the cosmetic cake. For younger patients, injection of fillers can serve as the sole procedure used to disguise aging; for older patients, fillers can be used with other rejuvenative procedures to refine treatment results.
Over the millennia, various substances have been injected into the face, including wax, silicone, and animal products ( Table 29-1 ).
| 1893 | Neuberg | First to use autologous fat for tissue augmentation |
| 1899 | Gersvny | First to use bioinjectable paraffin to correct cosmetic deformities |
| 1910 | Lexor | First to use large block grafts to treat malar depression |
| 1911 | Burnings | First to describe transfer of free fat with syringe technique |
| 1953 | Baronders | Used liquid silicone in medicine |
| 1959 | Peer | Reported 50% survival of syringe-aspirated transplanted fat for 1 year |
| 1976 | Fischer | Suction extraction of fat |
| 1978 | Illouz | Liposuction as fat source |
| 1981 | FDA approval of Zyderm | |
| 1986 | Fornier | Microliposuction |
| 2000 | FDA approval of Restylane, the first non-animal, hyaluronic acid filler |
Contemporary cosmetic facial surgery provides many options for augmenting lips, folds, and wrinkles. For decades, bovine collagen has been the “gold standard” for facial filler augmentation in the United States. Our European, Canadian, and Australian neighbors have been more proactive in the use of various fillers ; this technology is just reaching our shores and this, in part, is responsible for the enormous media coverage of injectable filler substances.
RECENT HISTORICAL OVERVIEW OF FACIAL FILLERS AVAILABLE IN THE UNITED STATES
An overview of injectable filler substances can be confusing because more than 120 filler products or devices are available. Many options exist, as do many fillers, many substances, many materials, and many claims of superiority. This chapter cannot cover all available fillers in depth and will concentrate on those commonly used (or soon to be released for use). As stated earlier, bovine collagen (Zyplast, Zyderm; Inamed Corp., Santa Barbara, Calif) dominated the U.S. market for longer than 2 decades. These fillers contained lidocaine, which mitigated the discomfort of the injection process. Because these products were of bovine derivation, allergy testing was a prerequisite. Classically, patients were inoculated in the forearm with the material, and, if no local allergic response was seen after 30 days, the material was assumed to be safe. Some practitioners advocated two successive monthly negative allergy tests. The need for testing proved to be a great drawback because many cosmetic consumers are spontaneous and want immediate treatment.
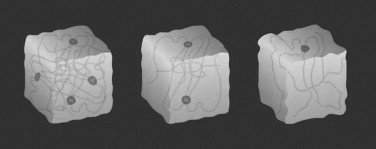
A foreign invasion of injectable fillers has occurred since December 2003. At the end of 2003, Restylane (hyaluronic acid; Qmed, Uppsala, Sweden), which has been used in many countries for over a decade, received U.S. Food and Drug Administration (FDA) approval. This marketing release brought an onslaught of media attention that boosted the entire perception of and desire for fillers by aging baby boomers. Restylane changed the paradigm in this country for injectable fillers for numerous reasons. First, it is a non-animal product synthesized from bacteria. Hyaluronic acid is a highly hydrophilic polysaccharide found in all living cells that attracts and binds more than 1,000 times its weight in water. Hyaluronic acid is chemically, physically, and biologically similar in all species. Because of this, there is no reason for allergy testing, which was one of the biggest drawbacks of bovine collagen products. Second, studies show that Restylane lasts longer than Zyplast. The longevity of Zyplast has long been a problem for patients and injectors. Although the product was easy to use and produced acceptable results, its effects lasted only several months in most patients, whereas some studies showed that Restylane can last up to 8 months. One of the reasons for extended longevity with the hyalurons (hyaluronic acid fillers) is a process called isovolemic degradation. Normally, collagen fillers are simply phagocytized and degraded, and this causes a decrease in volume. In this process, water is drawn into the filler of the hydrophilic molecule as the filler degrades. In this way, filler volume is retained longer as more water is continually drawn into the filler molecule ( Figure 29-1 ).
Multiple studies have shown Restylane to be a safe and effective facial filler. The product line includes Perlane, which has larger particle sizes and is used for deep dermis and subcutaneous injection, Restylane SubQ for subcutaneous injection, and Restylane Fine Line, which has the smallest particle sizes and is indicated for superficial dermal injection for fine lines and wrinkles.
In 2003, Inamed Aesthetics (Santa Barbara, Calif) introduced CosmoPlast and CosmoDerm, which are human collagen derivatives produced from tissue-cultured human infant foreskin. CosmoDerm and CosmoPlast collagen-based dermal fillers are sterile devices composed of highly purified human-based collagen that is dispersed in phosphate-buffered physiologic saline containing 0.3% lidocaine. CosmoDerm is a non–cross-linked formulation that is used in the treatment of superficial lines, whereas CosmoPlast is cross-linked and is used primarily in the treatment of more pronounced wrinkles. CosmoDerm is a purified human-based collagen 35 mg/mL, dispersed in saline. It is indicated to treat fine lines, wrinkles, and shallows scars. CosmoPlast is a purified human-based collagen 35 mg/mL, cross-linked with glutaraldehyde dispersed in saline. It is indicated to treat deep lines, furrows, and scars. Because these products are of non-animal origin, allergy testing is not necessary. Although they are very easy to inject because they have excellent flow properties, the author has found these products to possess similar longevity to that of bovine collagen predecessors.
Hylaform (Inamed) gained FDA approval in 2004 and has competed with Restylane in the new filler arena. Hylaform is a sterile, non-pyrogenic, viscoelastic, clear, colorless gel implant composed of cross-linked molecules of hyaluronan. Hyaluronan is a naturally occurring polysaccharide of the extracellular matrix in human tissues, including skin. Differences lie in the fact that this hyaluronic acid product is derived from animals (rooster combs) and contains less hyaluronic acid per milliliter than Restylane. The concern with avian flu has caused some patients to be concerned. Hylaform Plus and Captique were introduced by Inamed in 2004. These fillers took a relative back seat to Restylane, which has led the pack since its introduction. On March 23, 2006, Allergan, Inc. acquired Inamed Corporation, and Inamed is now a wholly owned subsidiary of Allergan. In June 2005, the FDA approved a new (to this country) hyaluronic acid–based filler line called Juvederm. The three product formulations include Juvederm 24HV, a highly cross-linked formulation that provides greater versatility in contouring and volumizing of facial wrinkles and folds; Juvederm 30HV dermal filler, a more highly cross-linked robust formulation for volumizing and correction of deeper folds and wrinkles; and Juvederm 30, a highly cross-linked formulation used for subtle correction of facial wrinkles and folds.
On February 28, 2003, the General and Plastic Surgery Devices Advisory Panel of the FDA recommended that ArteFill be approved, along with conditions for marketing in the United States. ArteFill, which is expected to become the first permanent esthetic injectable implant to gain FDA approval, is a combination of homogeneous precision-filtered microspheres suspended in a solution of purified collagen gel and 0.3% lidocaine, which is included to alleviate discomfort during injection. ArteFill has been designed with dual action to correct facial wrinkles. ArteFill does this as a result of its composition, which is a combination of precision-filtered microspheres (20% of total volume) made from polymethylmethacrylate (PMMA) and purified bovine collagen (80% of total volume). All microspheres have a defined size of 30 to 50 microns in diameter and have a smooth, round surface. Esthetic results are visible immediately after injection. PMMA is not taken up by scavenger cells (macrophages) and cannot be degraded by enzymes. Thus, the microspheres remain intact beneath the creases, providing a permanent support structure to support the wrinkle and prevent further wrinkling. As with all products in which bovine collagen is used, a skin sensitivity test must be performed prior to use.
Oral and maxillofacial surgeons have used hydroxyapatite products for augmentation for the past 20 years. Radiesse (formerly Radiance FN; BioForm, Franksville, Wis) is an injectable filler that consists of hydroxyapatite microspheres in a soluble gel vehicle ( Figure 29-2 ).
This product was developed for vocal cord augmentation and as a radiopaque soft tissue marker but was used off-label for soft tissue filling. The author uses Radiance FN when requested by patients, primarily in the nasolabial folds. The flow properties of Radiance FN are different from those of other fillers. The most noticeable property of Radiance FN is that a little product goes a long way. Because this product is hydroxyapatite based, the longevity is increased. Overfill or asymmetry can be a problem because it persists for a long time. The author does not recommend this product for the novice injector. Because Radiance FN is opaque, lip injection is visible on radiographs, and patients and their dentists should be made aware of this.
Finally, a plethora of new products that have been used in other countries are “knocking at the door” of the FDA. In 2005, NewFill, which has been used abroad, was FDA approved for the treatment of human immunodeficiency virus (HIV)-associated facial lipoatrophy. In the United States, this product is called Sculptra (Aventis Pharmaceuticals, Bridgewater, NJ). Patients with HIV are living longer and healthier lives because of the advent of new antiretroviral drugs. A side effect of these medications is lipoatrophy of the temporal and facial regions. This stigma makes HIV-positive patients stand out and has many negative social implications. Although this condition is amenable to treatment with multiple fillers, Sculptra has become a popular option. Sculptra is an injectable implant that contains microparticles of poly-L-lactic acid, a biocompatible, biodegradable, synthetic polymer from the alpha-hydroxy acid family. Sculptra is reconstituted prior to use by the addition of sterile water for injection (USP SWFI) to form a sterile non-pyrogenic suspension ( Figure 29-3 ).
Sculptra is intended for restoration and/or for correction of the signs of facial fat loss (lipoatrophy) in people with HIV. Many practitioners use Sculptra off-label to fill lines and wrinkles. The mechanism of Sculptra is different from that of other fillers in that the response is not immediate, but rather poly-L-lactic acid particles serve to initiate an inflammatory reaction and induce the production of collagen in the area. A typical treatment course for severe facial fat loss consists of three to six injection sessions, with the sessions separated by 2 or more weeks. The full effects of the treatment course are evident within weeks to months. Patients should be reevaluated no sooner than 2 weeks after each injection session to determine whether additional correction is needed. Patients should be advised that supplemental injection sessions may be required to maintain an optimal treatment effect.
Autogenous fat, dermis, and patient-cultured tissue fillers and fascia can be used as tissue fillers, but this discussion is beyond the scope of this chapter. Injectable silicone, a filler of historic significance that has earned praise and scorn over the past 50 years, is discussed separately in this chapter.
TISSUE POSITIONING OF INJECTABLE FILLERS
It is far more important to know how the filler is used than to know which filler is used. In other words, a good injector can get good results from any filler if he or she knows how to use it and where to put it, whereas an inexperienced injector can use the premium filler and obtain poor results.
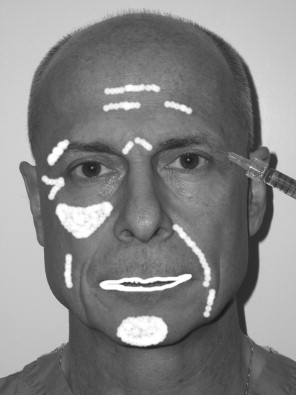
The key to placing any and all fillers is that specific injection techniques are required for correct placement in the skin and various anatomic sites. Figure 29-4 shows common areas of filler injection. Most fillers are specifically designed to be placed at a certain level ( Figure 29-5 ).
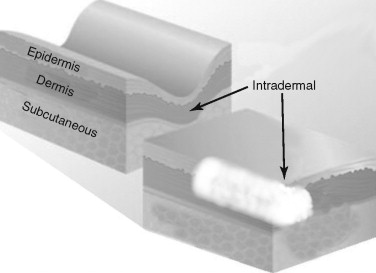
Restylane, for instance, is available in multiple products (Perlane, Restylane, and Restylane Fine Line) that vary in particle size. For this reason, the longevity and clinical results of the filler are based on the approximate level of tissue placement. If a fine-particle product like Restylane Fine Line is placed in the deep dermis or the subcutaneous (subQ) layer, the product will be degraded at a faster rate. By the same token, placement of a large-particle filler like Perlane in the superficial dermis will produce a lumpy result. Although some overlap is apparent, most fillers can be separated into dermal fillers or subQ fillers. Dermal fillers may be used specifically in the superficial, mid, or deep dermis. Zyplast, Cosmoplast, Restylane Fine Line, and Juvederm 30 are fillers that are designed for superficial dermal injection for fine lines and wrinkles. Restylane, Captique, and Juvederm 30 HV are more robust and are designed for deep dermal filling. Radiesse, Sculptra, silicone, and Arefill are designed for subcutaneous filling. The reader must understand that these classifications are generalizations, and that some practitioners use multiple fillers to layer specific areas and they use these fillers in whatever plane they desire. Experienced injectors have many tricks up their sleeves for individualized treatment. In addition, many of these product lines change the names of European products for use in the United States. As stated earlier, fillers are a complex subject, but once the doctor understands what they do, where to put them, and how to use them, it all boils down to a relatively simple algorithm.
TISSUE POSITIONING OF INJECTABLE FILLERS
It is far more important to know how the filler is used than to know which filler is used. In other words, a good injector can get good results from any filler if he or she knows how to use it and where to put it, whereas an inexperienced injector can use the premium filler and obtain poor results.
The key to placing any and all fillers is that specific injection techniques are required for correct placement in the skin and various anatomic sites. Figure 29-4 shows common areas of filler injection. Most fillers are specifically designed to be placed at a certain level ( Figure 29-5 ).
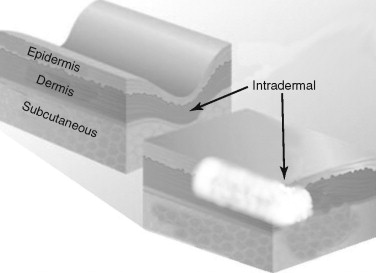
Restylane, for instance, is available in multiple products (Perlane, Restylane, and Restylane Fine Line) that vary in particle size. For this reason, the longevity and clinical results of the filler are based on the approximate level of tissue placement. If a fine-particle product like Restylane Fine Line is placed in the deep dermis or the subcutaneous (subQ) layer, the product will be degraded at a faster rate. By the same token, placement of a large-particle filler like Perlane in the superficial dermis will produce a lumpy result. Although some overlap is apparent, most fillers can be separated into dermal fillers or subQ fillers. Dermal fillers may be used specifically in the superficial, mid, or deep dermis. Zyplast, Cosmoplast, Restylane Fine Line, and Juvederm 30 are fillers that are designed for superficial dermal injection for fine lines and wrinkles. Restylane, Captique, and Juvederm 30 HV are more robust and are designed for deep dermal filling. Radiesse, Sculptra, silicone, and Arefill are designed for subcutaneous filling. The reader must understand that these classifications are generalizations, and that some practitioners use multiple fillers to layer specific areas and they use these fillers in whatever plane they desire. Experienced injectors have many tricks up their sleeves for individualized treatment. In addition, many of these product lines change the names of European products for use in the United States. As stated earlier, fillers are a complex subject, but once the doctor understands what they do, where to put them, and how to use them, it all boils down to a relatively simple algorithm.
INJECTABLE FILLERS: TREATMENT CONSIDERATIONS
Doctor, How long will my filler last? This is a question asked by many patients that is not an easy one to answer. For sure, the newer fillers outlast Zyplast and previous collagen fillers. Although companies quote slightly optimistic lengths of time, all are variable. Longevity depends upon the type of filler, the patient’s metabolism, the area of the face in which the filler is placed, and other variables. As a general rule, fillers placed in areas of extreme motion such as the lips will not last as long as those placed in more immobile areas such as the zygoma. Silicone will last forever, and Radiesse may last up to a year. This author makes no guarantees to his patients but merely explains that the new fillers last longer than the previous ones and a six-month maintenance result may be attained. Although the industry is pushing permanent fillers, it must be remembered that “permanent fillers can cause permanent complications!” When a filler is not liked by the patient or the surgeon, resorption is a good thing.
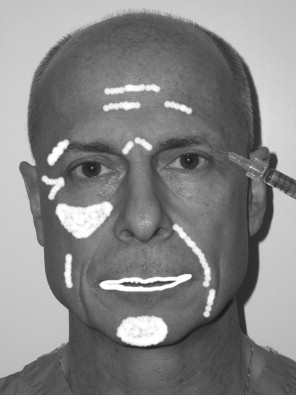
Currently, in the author’s practice, injection of various lip fillers is a daily occurrence. The default filler used is Restylane; however, many patients present with a specific request for a filler that they desire. Because of this tendency for patient preference, the author also injects Cosmoplast, Cosmoderm, Radiance FN, Sculptra, Juvederm, silicone, and fat. The most common requested site is the lips, followed by the nasolabial folds, the perioral region, cheek wrinkles, and “crow’s feet” wrinkles.
Because of the marketing hype, some patients confuse fillers with Botox. In addition, some patients desire massive rhytid injection, but because they have such a large amount of wrinkling, this would not be practical. These patients are informed that they would be better treated with lifting or resurfacing procedures. In theory, fillers can be injected anywhere on the face; however, blindness has been reported with periorbital injection of Zyplast and fat because of intravascular injection. This rare but devastating complication calls attention to the care that must be exercised in this area. The surgeon should always inject very superficially, use the smallest-gauge needle possible, and never use extreme plunger pressure on the syringe. Although the injection of fillers is simple, many problems can result in terms of patient expectations and satisfaction. The main consideration is to accurately explain what the patient can expect as a treatment result. Because many patients have been “media victims,” they present with unrealistic expectations, hoping for a miracle. Showing patients a series of before and after images for specific anatomic areas is one way to provide a reasonable expectation. In addition, the injection of fillers should not be presented as a one-time procedure but as a treatment sequence intended to gradually approach a result. Especially for some of the newer fillers such as Restylane that cause immediate swelling in the lips, judging the end-point and symmetry can be difficult ( Figure 29-6 ).
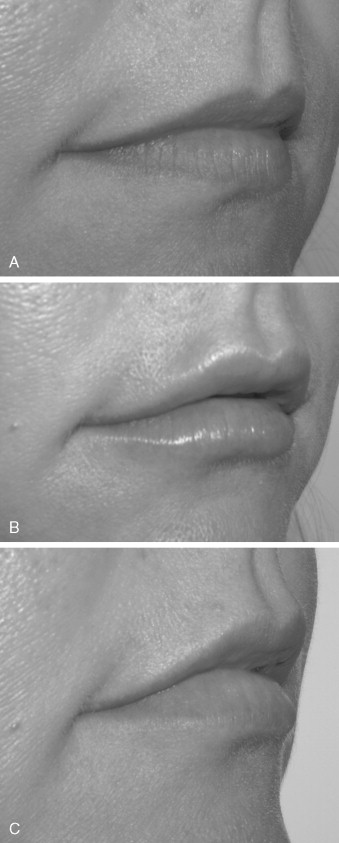
Having the patient return in 1 to 2 weeks gives the surgeon and the patient an opportunity to critique the result and to correct any areas of underfill or asymmetry. It also should be stated that any treatment should be conservative because more filler can easily be added. When the treatment area is overfilled or uneven, however, there is not much that can be done. Also paramount to communication is who will pay for the touch-up should it be necessary. Many means of working out this scenario are available, but the bottom line is that the method must be decided in the informed consent before treatment begins. The question of how much to use frequently arises. The novice injector or the novice patient may be unaware of what to expect in terms of how much area can be effectively treated with a single syringe. Again, the surgeon must be cognizant of how far a single syringe can go. When in doubt, it is prudent to pay attention to the amount left in the syringe, and when 50% has been used, the other side must be treated. Failure to do this will require opening of a second syringe, and if the patient was not expecting another $500 fee, an unpleasant discussion may follow. When in doubt, the patient should be told that a single syringe may not be adequate for the given augmentation.
TREATING THE LIPS
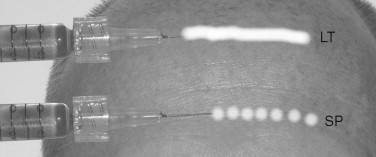
It is a good idea to use a surgical marking pen to outline the areas to be injected. These include lips, nasolabial folds, and wrinkles. By doing this, accuracy can be maintained in the face of interinjection edema, and the injector will not lose his or her landmarks. Every injector has his or her way of injecting fillers, but two recognized techniques are used universally. Linear threading involves inserting the needle and the injection filler as a straight line while continuously moving in a forward or backward direction. This process would be analogous to placing a line of toothpaste on one’s toothbrush. The other injection method is known as the serial puncture technique. This involves placing small boluses of filler with multiple punctures along the lip or wrinkle. The wrinkle is filled by deposition of the small bolus of filler along the wrinkle; multiple needlesticks are required ( Figure 29-7 ).
ANESTHETIC CONSIDERATIONS
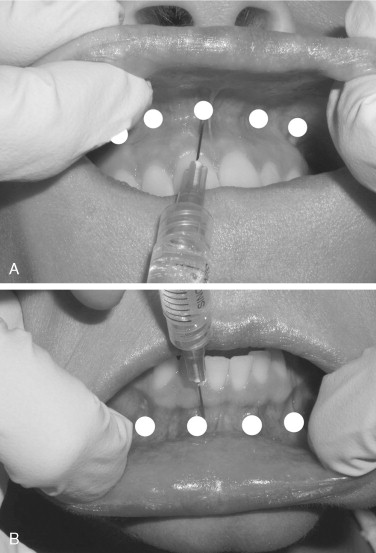
Most of the new fillers do not contain any inherent local anesthesia. The author strongly recommends using local anesthetic techniques when treating the lips. Many patients can tolerate filler injections in cutaneous areas such as the nasolabial folds or cheeks, but injecting the lips can provide significant discomfort. In addition, new and potent topical anesthetics are available. BLT cream (20% benzocaine, 6% lidocaine, and 4% tetracaine in a cream vehicle; Bayview Pharmacy, Baltimore, Md) is applied to the lips and the vestibular mucosa. When the upper lip is injected, bilateral infraorbital blocks are administered; bilateral mental blocks are given for the lower lips. Alternatively, the upper and lower vestibule can be infiltrated with three or four equally spaced injections from the cuspid area on one side to the cuspid area on the other side ( Figure 29-8 ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


