Fig. 3.1
Schematic representation of the important interactions between the immune system of the host, and bacterial microbiota and C. albicans in the GI tract. The GI microbiota produce several short-chain fatty acids known as inhibitors of the growth of C. albicans, but secrete parallel muramyl dipeptides (MDPs) that improve various virulence factors, including morphological transformation of yeasts to hyphae. The activation of Th17 cells results in the recruitment of neutrophils and macrophages, which share the ability to neutralize C. albicans. Macrophages, through the production of prostaglandin E2, prevent the differentiation of Th1 cells in favor of Th2 cells. Dendritic cells play a key role in the differentiation of various types of cells (Treg, Th1, Th2, Th17, and Th22). B lymphocytes, activated by IgA-producing Th2 cells, and epithelial cells, in response to Th22, release defensin. Fonte: Fabien Cottier • Norman Pavelka (Cottier and Pavelka 2012)
It is not clear how the host response contributes to exacerbation of the fungal infection. However, it is known that it depends on a number of interrelated factors. For example, there are inconsistencies regarding the role of the fungus interactions in inflammation in the host. On one hand, animals that do not have receptors for IL-1, IL-18, or NLRP3 exhibit increased susceptibility to fungal infection in the GI tract. On the other hand, exaggerated inflammatory responses are not associated with increased protection, but rather increased susceptibility to fungal infection, as is seen in patients suffering from immune reconstitution inflammatory syndrome (IRIS) (Singh and Perfect 2007). These apparently contradictory observations point to the fact that the host response to fungal infections does not follow a linear and predictable path, but it is suggested that there is a more complex balance in place to ensure control of the fungal burden and restrict damage to the host tissue (Romani 1999, 2011).
There are barriers, which are comprised of epithelial skin cells that are very close to each other, that block the passage of microorganisms. The cells of the GI tract produce mucus that physically prevents fungal invasion, and removal is possible by the action of intestinal peristalsis (Romani 1999, Romani 2004).
Oral Candidiasis
Oral candidiasis is a common opportunistic infection of the oral cavity and presents similar challenges in both immunologically competent and immunodeficient patients (Samaranayake et al., 2009). Studies have reported that 90 % of patients with acquired immunodeficiency (i.e., HIV) infection develop oropharyngeal, which is characterized by lesions that are pseudomembranous, erythematous or angular cheilitis, during the course of the disease (Repentigny et al., 2004). About 80 % of all fungal infections are caused by Candida, typically C. albicans. However, non-albicans Candida, such as C. glabrata, C. tropicalis, C. parapsilosis, C. Guilliermondii, and C. krusei, are also pathogenic to humans and have emerged as important opportunistic pathogens of the oral mucosa (Li et al., 2007).
Studies have focused on the interaction of C. albicans with macrophages and systemic infections (Herre et al., 2004; Mencacci et al., 2000). Although little is known about the performance of the oral mucosa in the regulation of fungal infections, some studies have contributed to the understanding of pathogen recognition and cell signaling mechanisms in oral epithelium. C. albicans interacts with epithelial cells through adhesion, invasion, and induction of cellular damage (Fig. 3.2).
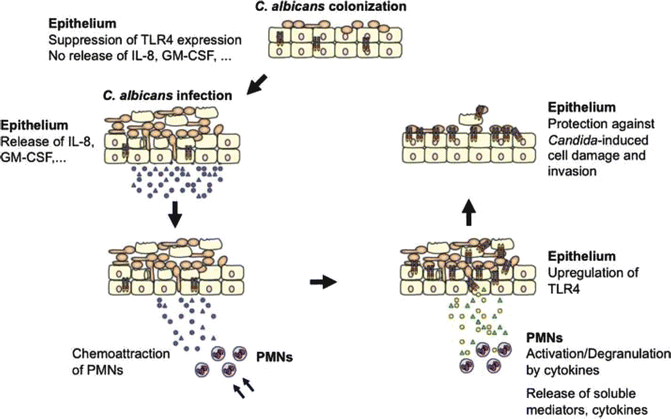

Fig. 3.2
Model of TLR4-mediated and PMN-dependent antifungal defense by the oral epithelium. In general, experimental oral infections can be divided into three phases: an attachment phase, an invasion phase, and a tissue destruction phase. Although C. albicans normally exists as a yeast cell, adherent yeast cells rapidly form germ tubes after contact with epithelial cells, and hyphae penetrate the epithelium. Tissue damage is increased dramatically over time, as hyphae penetrate not only the top layer of tissue, but also deeper epithelial cell layers. In contrast, epithelial cells control fungal cell growth and invasion. During colonization of the oral epithelium, C. albicans suppresses TLR4 expression and does not induce cytokine production. Infection, particularly in predisposed patients, leads to increased cytokine secretion that recruits and stimulates PMNs at the site of infection. After recruitment, several cytokines, especially TNF, are directly involved in initiating the subsequent PMN-mediated upregulation of epithelial TLR4 through a process that does not require PMN infiltration of the mucosal tissues. Finally, epithelial TLR4 directly protects the oral mucosa from fungal invasion and cell injury, possibly by production of antimicrobial peptides. Fonte: G. Weindl, J. Wagener, and M. Schaller (Weindl et al., 2010)
Several advances have been reported in the literature that help to better explain the role of receptor signaling and regulation of immune response against pathogenic fungi in the oral cavity (Graham and Brown 2009; Mencacci et al., 1996). Innate immunity also plays an important role in stimulating the acquired immune response and improves its action against various microorganisms. Innate immunity, which is the first line of defense against microorganisms, includes leukocytes and epithelial barriers.
The interaction between fungi and the host is initiated by cell–cell interactions at the plasma membrane. Several different molecules are found in the walls of fungal cells and serve as molecular patterns (i.e., PAMPs) that are associated with specific pathogens. The PAMPs are recognized by PRRs. The cell wall of most fungi, including C. albicans, is composed of a layer of β-(1,3)-glucans linked to β-(1,6)-glucans and chitin, in which the cell wall proteins, and O-linked and N-linked mannans are incorporated. In addition, there are receptors that recognize molecules synthesized by the host, the expression of which indicates cellular damage, known as DAMP (Damage Associated Molecular Patterns).
Mammals have developed a series of PRRs capable of recognizing different molecular fragments that compose the fungal cell wall. PAMPs (Pathogen-associated molecular patterns) for fungi and PRR (Pattern Recognition Receptor) include Toll-like receptors (TLR), C-type lectin receptors (CLRs), Nod-like receptors (NLR) (nucleotide-binding oligomerization domain), and galectin family proteins (Graham and Brown 2009; La Sala et al., 1996). TLRs are a family of evolutionarily conserved signaling receptors that respond to bacterial antigens, fungal and viral. They are found on the cell surface and intracellular membranes and, thus, are capable of recognizing microorganisms different cellular locations intracellular. TLRs induce, among other cellular responses, innate immunity, the production of cytokines, chemokines, and endothelial adhesion molecules. They have the ability to recognize a variety of microbial antigens and endogenous factors, presented well, the primary function of acting as receptors sentry to alert the innate immune system signs of infection or tissue damage (Netea et al., 2008).
TLR are expressed on macrophages, dendritic cells, neutrophils, endothelial, and epithelial cells of mucous membranes (Takeda et al., 2003). TLR2, TLR4, TLR6, and TLR9 have been implicated in interactions with fungal sens[aory PAMPs, such as mannans O-linked β-glucans and fungal nucleic acids (Miyazato et al., 2009; Romani et al., 1997).
The main PRRs and their ligands derived from C. albicans are shown in Fig. 3.3. Although the main focus of antifungal innate immunity research has been on systemic Candida infections, little is known about the role of TLRs in fungal infections located. From studies of fungal infections in knock-out mice deficient in either TLRs or TLR-associated adapter molecules, it appears that specific TLRs, such as TLR2, TLR4, TLR6, and TLR9, play different roles in the activation of the innate immune response. Different research groups have demonstrated divergent roles for TLR2 and TLR4, and their importance in the control of C. albicans infection is still uncertain.
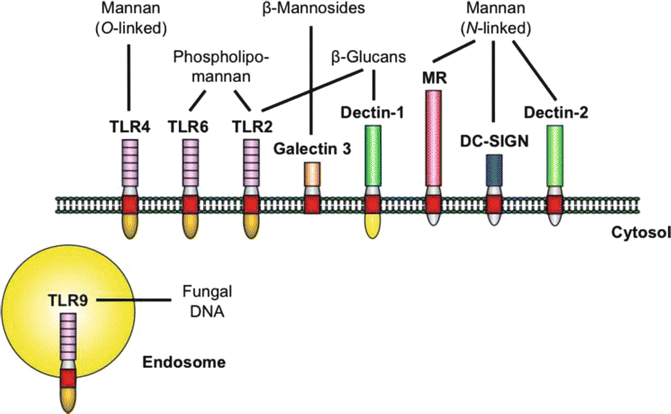

Fig. 3.3
The major PRRs involved in the recognition of specific C. albicans PAMPs. Stimulation of the host response by C. albicans at the cell membrane is mediated by a limited number of PRRs from the TLR and CLR families. Upon activation, the receptors trigger common adaptor molecules, intracellular pathways, and transcription factors (not shown). However, the specificity of the host response is maintained by the different repertoire of receptors stimulated by certain fungal PAMPs, as well as by the complex interactions between the pathways. The depicted PRRs are predominantly expressed on cells of the myeloid lineage. Oral epithelial cells have been shown to express functional TLR2, TLR4, TLR6, and TLR9, emphasizing the importance of TLRs in the interaction of C. albicans and the oral epithelium
NLRs, including Nod1, Nod2, and Nod3 are found intracellularly and recognize peptidoglycan derivatives, microbial cell wall components, and other intracellular signals leading to danger. They activate the host defense through activation of the NF-kB response and inflammatory caspases (Bryant and Fitzgerald 2009
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


