Introduction
Asthma is a common systemic disease occurring in infancy and adolescence, time periods that could encompass orthodontic treatment. Asthma is an inflammatory disease; therefore, it might interfere with orthodontic tooth movement. The purpose of the study was to analyze the histomorphologic aspects of the periodontal ligament of asthmatic Wistar rats in the initial period of orthodontic movement.
Methods
Thirty-two Wistar rats were divided into 4 groups: 2 control groups consisting of rats without induced allergic asthma, and 2 experimental groups consisting of rats with induced allergic asthma. The animals of the first control and experimental groups did not receive orthodontic forces, whereas those in the second control and experimental groups were subjected to mesial movement of the maxillary left first molar for 3 days. The samples were prepared for histomorphometric analysis of the periodontal ligament. The area of the periodontal ligament was calculated as a function of root length in the cervical and apical regions of the distal face of the maxillary first molar mesial root. The Student t test and the Welch correlation test were applied to the data obtained.
Results
There was a statistically significant difference ( P <0.05) between the control and experimental groups. An enhanced response to orthodontic force was observed in the asthmatic animals: the periodontal ligament was more compressed at the pressure area and more stretched in the traction area.
Conclusions
Our findings indicate that experimental allergic asthma seems to exacerbate orthodontic movement in rats.
Asthma is a disease of the airways characterized by hypersensibility of the tracheobronchial tree to several stimuli. This condition is evidenced by generalized narrowing of airways and can be alleviated spontaneously or with medication. Clinically, asthma manifests itself through dyspnea, coughing, and wheezing. Asthma is an episodic disease in which acute exacerbations are intercalated with asymptomatic periods. It can be unchained as a consequence of inhalation of domestic dust, hair, smoke, chalk powder, strong odors, or chemical sprays; ambient temperature changes; emotional disturbance; hyperventilation; physical exercise; and viral infection. Exposure to allergens, particularly during the first years of life, can determine the chronic inflammation of the airways of those who are genetically susceptible to asthma development. Asthma affects about 5% to 10% of the population, and its incidence has increased significantly during the last 20 years in industrialized countries.
Asthma’s etiology is complex and multi-factorial. There is an interaction between genetic factors and environmental stimuli contributing to the development of a mediated immune response, predominantly by activation of TH2 lymphocytes. Laboratory analysis of the bronchoalveolar lavage fluid from asthmatic patients shows inflammatory reactions characterized by increased amounts of mast cells, epithelial cells, eosinophils, lymphocytes, and inflammatory mediators. Once released, such mediators produce immediate and intense inflammatory reactions, involving bronchoconstriction, vascular congestion, and tissue edema. Generalized increases in cellularity and the amount of capillaries are the most frequent anomalies observed in asthma. Eosinophilia is the most common finding in the blood count.
Similarly to the asthma process, the inflammatory process is also found in the periodontum during the initial period of orthodontic movement. In the phase of acute inflammation, one can observe an increase in the caliber of blood vessels in the periodontal ligament and structural changes in this tissue. Therefore, soon after force application, a rapid and small dental displacement occurs for 4 to 7 days as a result of inflammatory changes and gradual compression of the periodontal ligament.
Such considerations support the purpose of our study: ie, to analyze the histomorphologic aspects of the periodontal ligament of asthmatic Wistar rats in the initial period of orthodontic movement. Because asthma is an inflammatory disease, it can interfere with orthodontic movement.
Material and methods
Male Wistar rats (180-200g) were obtained from the Oswaldo Cruz Foundation breeding center (Rio de Janeiro, Brazil). All procedures involving the care and use of laboratory animals in this study were approved by the Animal Ethics Committee of the Federal University of Rio de Janeiro (reference number, ODONTO 004).
The animals were divided into 2 groups: the control group consisting of 16 animals was actively sensitized and stimulated with saline solution, and the experimental group consisting of 16 animals was also actively sensitized and stimulated with antigen. In both groups, dental movement was conducted for 3 days in half of the animals, and the rest had no orthodontic intervention. This determined the establishment of the subgroups: control group 1, control group 2, experimental group 1, and experimental group 2 with 8 animals in each.
All the animals were sensitized through subcutaneous injection containing 50 μg of ovalbumin and 5 mg of aluminum hydroxide, for a total volume of 0.2 mL. After 14 days, the reinforcement procedure was carried out with an intraperitoneal injection of antigen solution. The antigenic challenge was performed only in the experimental groups through nasal instillation with the antigen (ovalbumin, 700 μg per animal) diluted in sterile saline solution of 0.1 mL in each animal. The animals of the control group were challenged with 0.1 mL of saline solution. Such challenges were performed for 3 consecutive days from day 7 after reinforcement. These procedures ensured the maintenance of basal metabolism in these animals. The orthodontic devices were installed in control group 2 and experimental group 2 on the day after the last challenge.
Blood samples were obtained from all animals. Total leukocytes were counted in a Neubauer chamber, and a differential analysis was performed under oil-immersion objective in blood samples and cytocentrifuged smears stained with May-Grunwald-Giemsa stain. Orthodontic devices were installed after verifying the increase in total leukocyte numbers in the experimental group compared with the control group. Differential blood cell counts were obtained from laboratory prepared slides containing blood smear stained by the May-Grunwald-Giemsa method. The slides were analyzed under a light microscope with oil-immersion objective lenses. The results obtained from the total leukocyte count and the differential blood cell count were submitted to the Student t test for nonpaired samples.
An intraperitoneal single dose of 40 mg per kilogram of sodium thiopental was administered for fitting the orthodontic device in all animals of control group 2 and experimental group 2. Mesial movement of the maxillary left first molar was obtained by attaching a nickel-titanium coil spring 7 mm in length and calibrated at 40 gf. The mandibular incisors had their height reduced with a high-speed dental hand piece to prevent damage to the orthodontic appliance. Only the animals in control group 2 and experimental group 2 were anesthetized and had the device installed and the mandibular incisors worn.
All animals were killed with a lethal intraperitoneal injection of sodium thiopental (60 mg/kg) 3 days after dental movement in experimental group 2 and control group 2. Soon after checking the loss of vital signs, bronchoalveolar lavage was performed by inserting a polyethylene cannula into the trachea for administration and aspiration of 15 mL of heparinized phosphate buffered saline (10 IU/mL) in 3 successive lavage procedures of 5 mL each. Bronchoalveolar lavage fluid was collected, and total leukocytes were counted as previously described for blood analysis. Differential counts were performed in cytocentrifuged smears stained with May-Grunwald-Giemsa stain.
Anatomic pieces containing the maxillary left first molars and lungs were fixed in 4% paraformaldehyde solution buffered with 0.1 mol per liter of sodium phosphate for 48 hours. The dental specimens were prepared with a decalcification method by using 40% formic acid and 20% sodium citrate solution for subsequent hematoxylin and eosin staining of the histologic sections. The dissected lungs were submitted to the classic histotechnical procedures with Giemsa staining after the fixation period.
Lung tissue sections were studied under a light microscope, with peribronchial eosinophils counted in 4 bronchi per animal. Section thickness was 5 μm. A morphometric analysis was conducted in 3 regions around each bronchus. The values for the control and experimental groups were submitted to the Student t test for nonpaired samples.
Histologic sections containing dental samples were evaluated under a light microscope (Eclipse; Nikon, Tokyo, Japan) for histomorphometric analysis of the periodontal ligaments. Section thickness was 5 μm. Four sections were measured per animal; only those that included the dental pulp were used to ensure the evaluation of deep histologic sections. Software (Image-Pro Plus, version 4.5; MediaCybernetics, Bethesda, Md) was used on the digital images obtained for measurement of the apical and cervical regions of the periodontal ligament on the distal face of the maxillary left first molar mesial root.
A standardized analytic method was adopted for reading and comparing the data between the subgroups. The methodology consisted of tracing 2 straight lines parallel to each other: 1 tangential to the furca, limiting the cervical point of the mesial root, and the other tangential to its most apical point, both parallel to the lower border of the photograph. Next, another straight line was traced perpendicular to both parallel lines, which was measured to determine root length. By dividing the root length by 3, the length of each root third was obtained. Once the cervical and apical thirds were delimited, the area of the periodontal ligament was calculated for the respective regions. To eliminate comparison errors resulting from individual characteristics of each animal, the ratio was calculated between the periodontal ligament area and its corresponding third length. The results were submitted to statistical analysis with the Student t test and the Welch correlation ( Fig ).
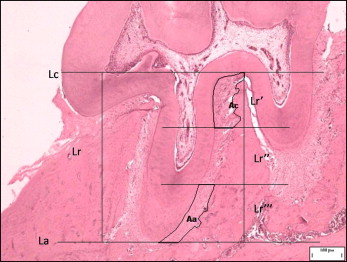
Results
Before fitting the orthodontic device, we first noted that the antigen challenge led to increases in the numbers of total leukocytes and eosinophils in the blood of experimental rats compared with the control animals. The experimental animals also showed significant increases in total leukocyte and eosinophil counts in the bronchoalveolar lavage ( Table I ). A marked leukocyte infiltration was observed in the lung parenchyma consisting mainly of eosinophils.
| Before installation of orthodontic device | |||
|---|---|---|---|
| Control group | Experimental group | ||
| Blood | Cells × 10 −3 /mm 3 | Cells × 10 −3 /mm 3 | P value |
| Total leukocytes | 88.1 ± 6.2 | 132.4 ± 8.7 | <0.05 |
| Eosinophils | 5.0 ± 1.2 | 16.8 ± 1.4 | <0.0001 |
| Before death | |||
|---|---|---|---|
| Blood | Cells × 10 −3 /mm 3 | Cells × 10 −3 /mm 3 | |
| Eosinophils | 1.0 ± 0.4 | 11.1 ± 1.0 | <0.0001 |
| After death | |||
|---|---|---|---|
| Bal | Cells × 10 5 /bal | Cells × 10 5 /bal | |
| Total leukocytes | 7.4 ± 0.8 | 10.4 ± 0.7 | <0.05 |
| Eosinophils | 0.1 ± 0.1 | 1.1 ± 0.2 | <0.05 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


