Introduction
“Periodontology” is the study of the tooth-supporting tissues, the “periodontium.” The periodontium is made up of those tissues that surround each tooth and which anchor each tooth into the alveolar process (Latin: para = adjacent to; Greek: odus = tooth).
The following soft and hard tissues constitute the structure of the periodontium:
|
• Gingiva |
• Periodontal Ligament |
|
• Root Cementum |
• Alveolar Bone |
The structure and function of these periodontal tissues have been extensively researched (Schroeder 1992). Knowledge of the interplay between and among the cellular and molecular components of the periodontium leads to optimum therapy, and also helps to establish the goals for future intensive research.
Periodontal Diseases
Gingivitis – Periodontitis
There are numerous diseases that affect the periodontium. By far the most important of these are plaque-associated gingivitis (gingival inflammation without attachment loss) and periodontitis (inflammation-associated loss of periodontal supporting tissues).
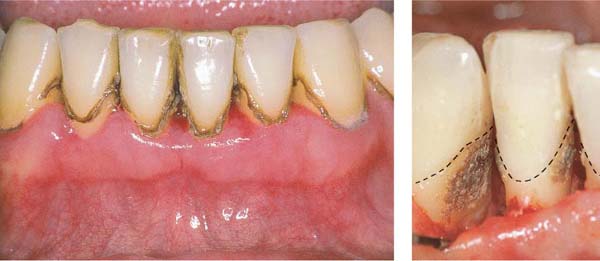
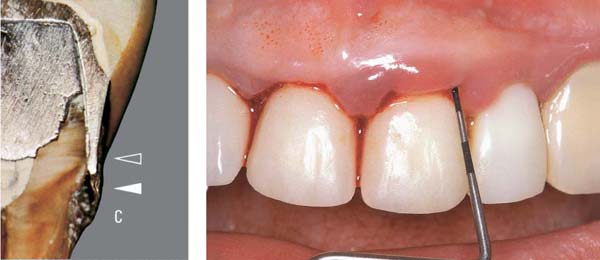
• Gingivitis is limited to the marginal, supracrestal soft tissues. It is manifested clinically by bleeding upon probing of the gingival sulcus, and in more severe cases by erythema and swelling, especially of the interdental papillae (Fig. 3).
• Periodontitis can develop from a pre-existing gingivitis in patients with compromised immune status, the presence of risk factors and pro-inflammatory mediators, as well as the presence of a predominately periodontopathic microbial flora. The inflammation of the gingiva may then extend into the deeper structures of the tooth-supporting apparatus. The consequences include destruction of collagen and loss of alveolar bone (attachment loss). The junctional epithelium degenerates into a “pocket” epithelium, which proliferates apically and laterally. A true periodontal pocket forms. Such a pocket is a predilection site and a reservoir for opportunistic, pathogenic bacteria; these bacteria sustain periodontitis and enhance the progression of the disease processes (Fig. 4).
Gingival Recession
Gingival recession is not actually a “disease,” but rather an anatomic alteration that is elicited by morphology, improper oral hygiene (aggressive scrubbing), and possibly functional overloading.
• Teeth are not lost due to classical gingival recession, but patients may experience cervical hypersensitivity and esthetic complications. If gingival recession extends to the mobile oral mucosa, adequate oral hygiene is often no longer possible. Secondary inflammation is the consequence.
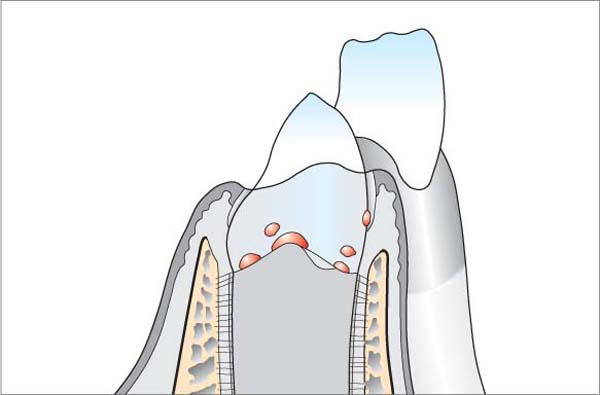
In addition to classical gingival recession, apical migration of the gingiva is often observed in patients with longstanding, untreated periodontitis, and it may be a consequence of periodontitis therapy in elderly patients (“involution”; Fig. 2).
These three periodontal disorders – gingivitis, periodontitis, gingival recession – are observed world-wide; they affect almost the entire population of the earth to greater or lesser degree. In addition to these common forms of oral pathology, there are many less frequently encountered diseases and defects of the periodontal tissues. All of these diseases were comprehensively classified at an international World Workshop in 1999 (see Appendix, p. 519).
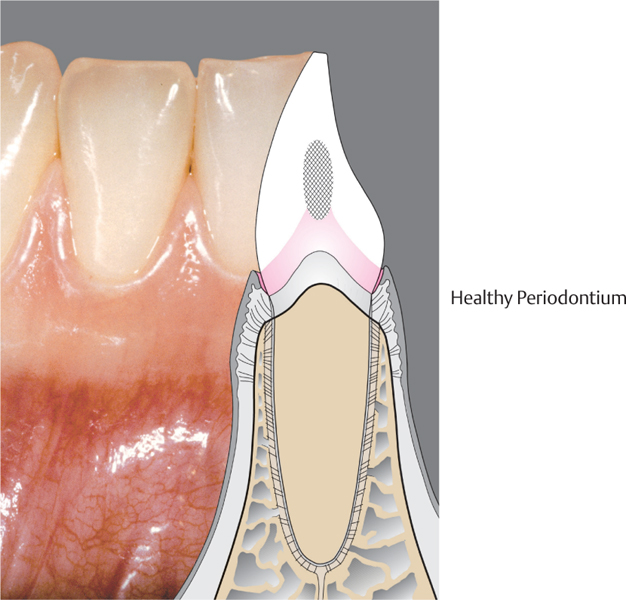
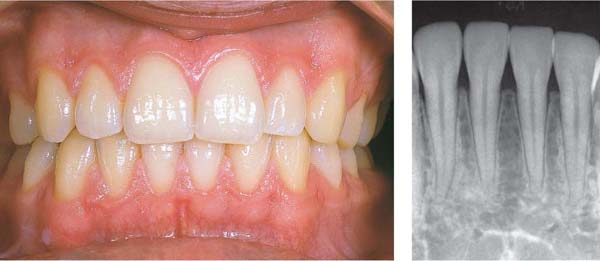
1 Healthy Periodontium
The most important characteristic of the periodontium is the special connection between soft and hard tissues:
• In the marginal region, one observes the inflammation-free gingiva, which provides the epithelial attachment to the tooth by means of its junctional epithelium (pink collar). This connection protects the deeper-lying components of the periodontium from mechanical and microbiologic insult.
• Subjacent to the junctional epithelium, one observes the supracrestal fibers, which serve to connect the tooth with the gingiva, and also the periodontal ligament fibers in the region of the alveolar bone, which insert into the bone and the cementum of the root surface.
Prevention of disease: Maintaining the health of the periodontium is the highest goal in periodontology, and should also be the patient’s goal. It is achieved by optimum, purely mechanical oral hygiene. Disinfectant mouth-washes may enhance mechanical hygiene.
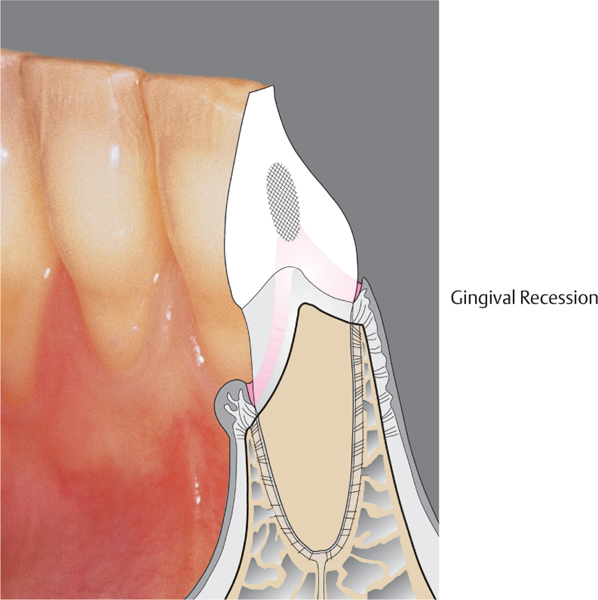
2 Gingival Recession
The main characteristic of this condition, which is often esthetically objectionable to patients, is an inflammation-free apical migration of the gingival margin. A morphological prerequisite is generally a facial bony lamella that is either extremely thin or entirely lacking. Gingival recession can be initiated and propagated by improper traumatic tooth brushing (horizontal scrubbing), and functional overloading may also play a role (?). Thus gingival recession cannot be classified as a true periodontal disease.
The best way for a patient to prevent gingival recession is by using an adequate but gentle oral hygiene technique (vertical-rotatory brushing or use of a sonic toothbrush).
Treatment: Incipient or progressing gingival recession can be halted by altering the patient’s oral hygiene techniques; in severe cases, mucogingival surgery may be employed to stop the progression or re-cover the exposed root surfaces.
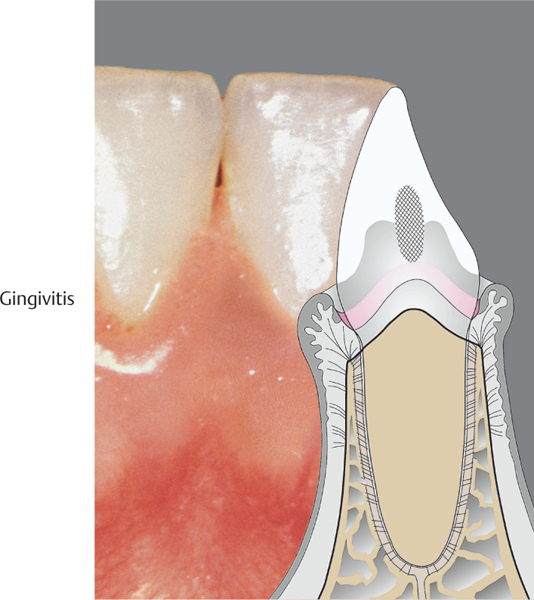
3 Gingivitis
Gingivitis is characterized by plaque-induced inflammation of the papillary and marginal gingivae. Clinical symptoms include bleeding on probing, erythema, and eventual swelling. Gingivitis may be more or less pronounced depending upon the plaque—a biofilm—(quantity/quality) and the host response. Deeper lying structures (alveolar bone, periodontal ligament) are not involved. Gingivitis may be a precursor to periodontitis, but this does not always occur.
Treatment: Gingivitis can be completely controlled simply through adequate plaque control. Following initiation or improvement of oral hygiene procedures, coupled with professional plaque and calculus removal, complete healing can be expected. Nevertheless, freedom from inflammation, e. g., absence of bleeding on probing, will be impossible to achieve if the patient is not capable of maintaining a high standard of oral hygiene over the long term, or is not willing to do so (compliance!).
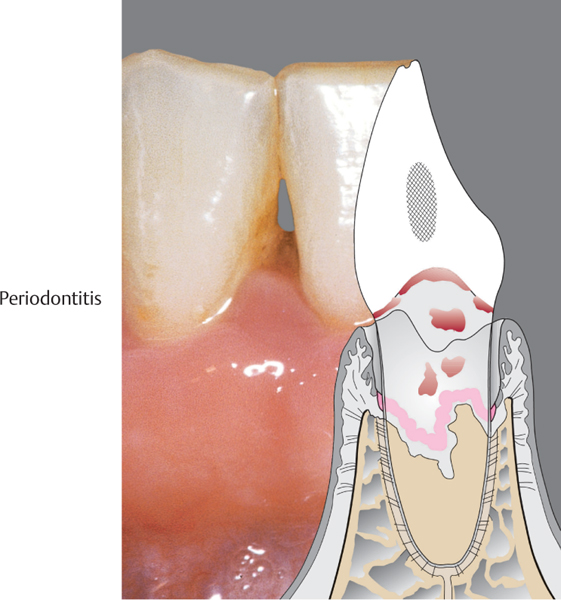
4 Periodontitis
At the gingival margin, the characteristics of periodontitis are similar to those of gingivitis, but the inflammatory processes extend further, into the deeper-lying periodontal structures (alveolar bone and periodontal ligament). True periodontal pockets are formed and connective tissue attachment is lost. Loss of hard and soft tissues is usually localized and not generalized.
Periodontitis may be classified as chronic (Type II) or aggressive (Type III), with varying degrees of severity. Approximately 90 % of all cases are characterized as “chronic periodontitis” (p. 108, 519).
Treatment: Most cases of periodontitis can be treated successfully. However, the required therapeutic endeavor can vary enormously from case to case. The treatment effort may be relatively small in early stages of periodontitis. Mechanical treatment remains today in the foreground. In special cases, topical and systemic medications may be used as supportive therapy.
The Clinical Course of Untreated Periodontitis
Periodontitis is usually a very slowly progressing disease (Locker & Leake 1993; Albandar et al. 1997), which in severe cases—particularly when untreated—can lead to tooth loss. Enormous variation in the speed of progression of periodontitis is observed when one differentiates between individual patients. In addition to the quantity and composition of the bacterial plaque, individually varying influences also play important roles: the systemic health of the patient, the patient’s genetic constitution, psychically influenced immune response status, ethnic and social factors, as well as risk factors such as smoking and stress (p. 22, Fig. 41). All of these circumstances can influence the onset and the speed at which the disease process accelerates in different patient age groups.
Not all teeth or individual surfaces of teeth are equally susceptible (Manser & Rateitschak 1996):
• Molars are the most endangered
• Premolars and anterior teeth are less susceptible
• Canines are the most resistant.
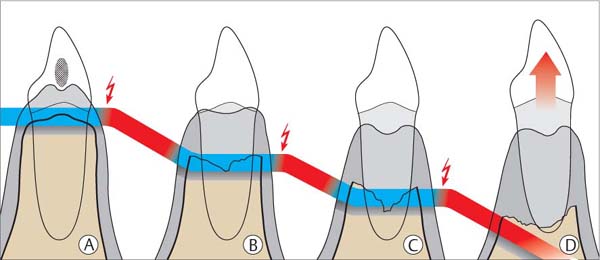
5 Clinical Course of Untreated Periodontitis
In the aggressive forms of periodontitis (pp. 95, 97), the manifestation of tissue loss on individual teeth occurs in successive acute phases rather than in a gradual, chronic progression. Phases of progression and quiescence alternate. Destructive phases may occur rapidly one after another, or longer quiescent phases may be in evidence.
|
Red |
Acute phase/destruction |
|
Blue |
Phase of quiescence |
Periodontitis—Concepts of Therapy
The primary goal is prevention of periodontal diseases, and the secondary goal is to enhance the healing of existent periodontitis, as far as possible toward complete Restitutio ad integrum. Clinical and basic research is targeted today toward realization of these goals in the near future.
At the present time, proven therapeutic concepts are available for the elimination of inflammation and the cessation of the progression of disease. In addition, to a certain degree, it is possible today to regenerate lost periodontal attachment (GTR, p. 338). The following treatment modalities are available to the periodontal therapist:
|
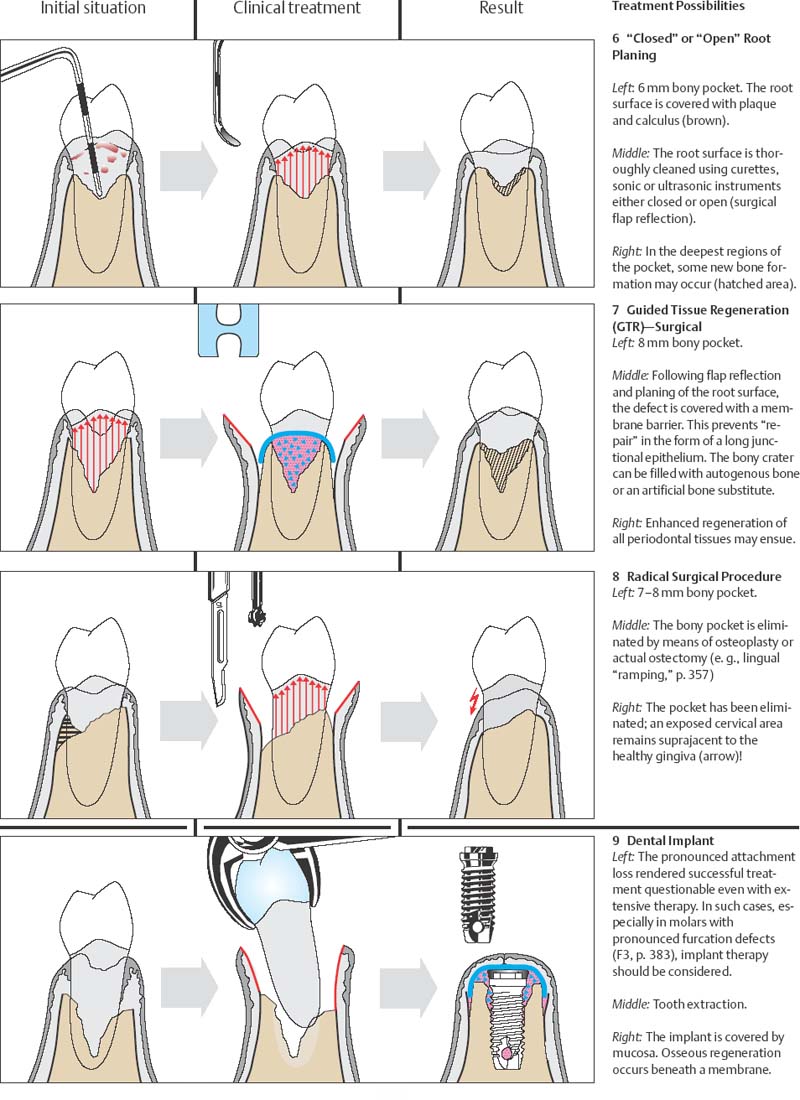
1 Closed or open root planing (“causal therapy,” the “gold standard”) 2 Regenerative surgical therapies 3 Resective surgical therapies 4 Alternatives: extraction or dental implants? |
1 Root planing is the Conditio sine qua non in all periodontal therapy. This is pure causal therapy, during which the causative “biofilm” (plaque) and subgingival calculus are removed. If the pockets are shallow and the morphological relationships simple (single-rooted teeth), treatment can be performed closed, but in advanced cases, open treatment with direct visual access following reflection of a tissue flap is often preferable (e. g., modified Widman procedure). The result of such treatment is usually healing in the sense of “repair” (p. 206). A long junctional epithelium forms.
2 Regenerative therapeutic methods (“guided tissue regeneration” [GTR]; autogenous, alloplastic implants) have taken on increased significance in recent years. These procedures are constantly being further developed, and in the future they may be enhanced by the use of growth and differentiation factors.
Regenerative therapy can lead to the actual re-formation of significant periodontal tissues.
3 Radical surgery for the elimination of periodontal pockets has lost some its popularity in recent years, although the results are generally more predictable and the tendency toward recurrence is low.
4 In cases of complex, severely advanced periodontitis, e. g., with severe furcation involvement in the molar region, the dentist may consider tooth extraction and replacement with a root-form implant instead of resective or regenerative periodontal surgery. Even in such cases, periodontal treatment of the remaining dentition must be performed, as well as optimum plaque control by the patient and the creation of an adequate amount of bone for the implant.
Structural Biology
“Structural biology” is a general term referring to the classical macromorphology and histology of tissues, as well as their function, including the biochemistry of the cells and the intercellular substances.
Basic knowledge of the normal structural biology of periodontal tissues and their dynamics (mediator-guided homeostasis, “turnover”) is a prerequisite for full understanding of pathobiological changes in the periodontium, which can involve adaptations of the normal structures or an imbalance of otherwise normal functions (Schroeder 1992).
The term “periodontium” encompasses four different soft and hard tissues: gingiva, root cementum, alveolar bone, and the periodontal ligament, which attaches root cementum to bone. Each of these four tissues can be further differentiated in terms of structure, function and localization.
10 Periodontal Structures
Left Side:
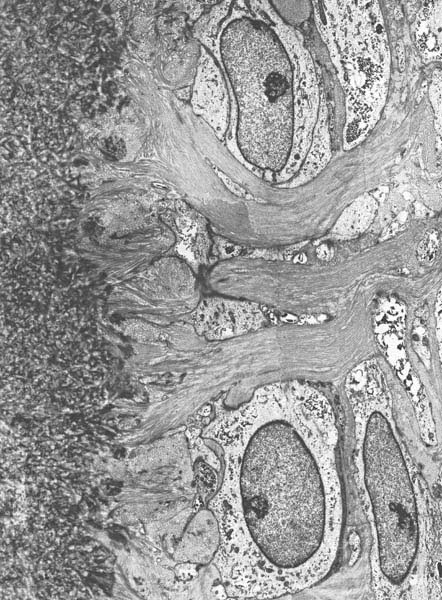
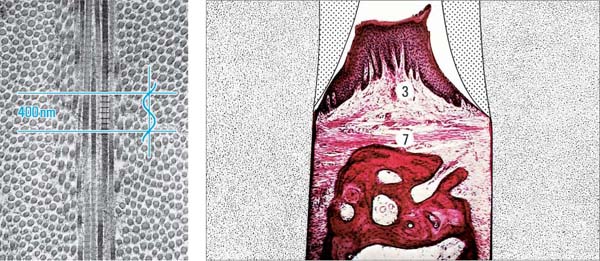
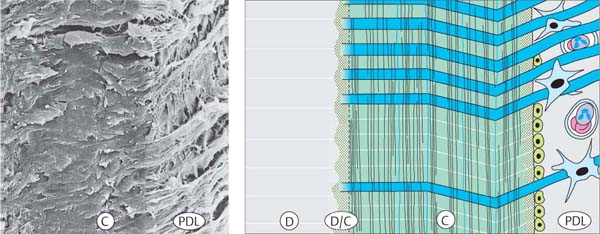
Transmission electron photomicrograph (TEM) of root formation in humans (ca. 6-year-old) This TEM depicts the growing demarcation between dentin, cementum and periodontal ligament during root formation. Initial mineralization of the “cementoid” directly apposed to the dentin, with penetrating collagen fibers and fibroblast-like cementoblasts, which are involved in the formation of acellular exogenous fiber cementum.
A Dentin
B Cementoid
C Radiating collagen fibers
D Cementoblast (fibroblast-like) building acellular exogenous fiber cement
Courtesy D. Bossardt, H. Schroeder
Gingiva
The gingiva is one portion of the oral mucosa. It is also the most peripheral component of the periodontium. Gingiva begins at the mucogingival line, and covers the coronal aspect of the alveolar process. On the palatal aspect, the mucogingival line is absent; here, the gingiva is a part of the keratinized, non-mobile palatal mucosa.
The gingiva ends at the cervix of each tooth, surrounds it, and forms there the epithelial attachment by means of a ring of specialized epithelial tissue (junctional epithelium; p. 10). Thus the gingiva provides for the continuity of the epithelial lining of the oral cavity.
The gingiva is demarcated clinically into the free marginal gingiva, ca. 1.5 mm wide; the attached gingiva, which may be of varying width; and the interdental gingiva.
Healthy gingiva is described as “salmon” pink in color; in Blacks (seldom also in Caucasians) the gingiva may exhibit varying degrees of brownish pigmentation. Gingiva exhibits varying consistency and is not mobile upon the underlying bone. The gingival surface is keratinized and may be firm, thick and deeply stippled (“thick phenotype”), or thin and scarcely stippled (“thin phenotype”; Müller & Eger 1996, Müller et al. 2000).
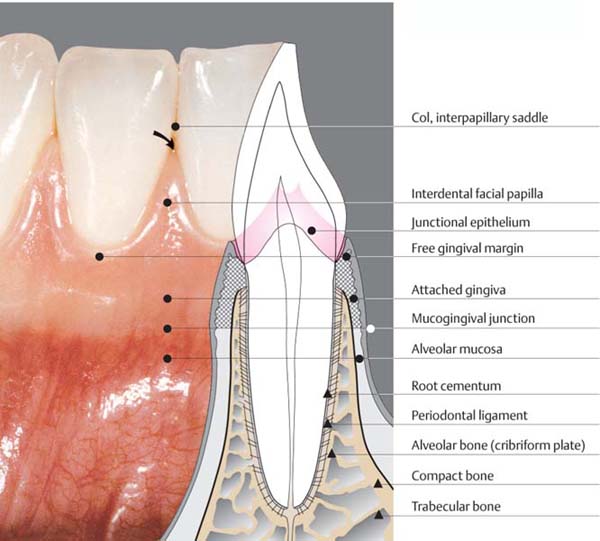
11 Healthy Gingiva
The free gingival margin courses parallel to the cementoenamal junction. The facial interdental papillae extend to the contact area of adjacent teeth. A gingival groove can be observed in some areas, demarcating the free gingival margin from the attached gingiva.
Right: The radiograph depicts normal interdental septa. In the original radiograph, the crest of the alveolar bone was observed ca. 1.5 mm apical to the CEJ.
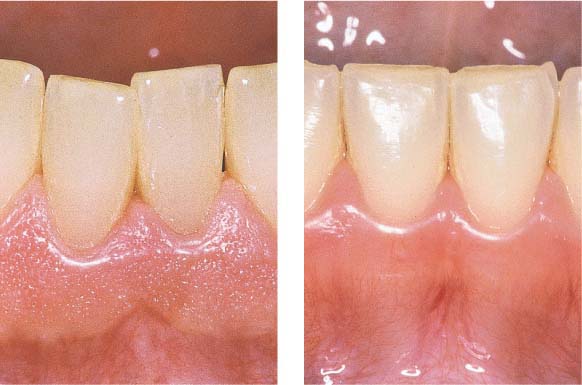
12 Variations in Consistency of Healthy Gingiva
Left: Firm, fibrous gingiva = “thick” phenotype.
Right: Delicate, scarcely stippled gingiva = “thin” phenotype. The subjacent bone covering the roots is clearly visible.
Thicker gingiva provides better conditions for treatment and wound healing (blood flow; stable position of the gingival margin).
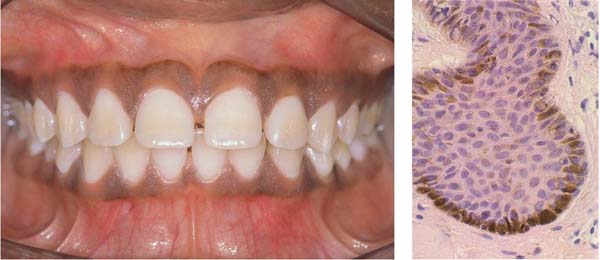
13 Healthy, Pigmented Gingiva
Note the symmetrical pigmentation of the attached gingiva in this 16-year-old African female.
Right: This pigmentation results from the synthesis of melanin by melanocytes located in the basal layer of the epithelium. The melanocytes in this histologic section appear as brown spots.
Gingival Width
The attached gingiva becomes wider as a patient ages (Ainamo et al. 1981). The width varies between individuals and among various groups of teeth in the same person. Although it was once believed that a minimum width of attached gingiva (ca. 2 mm) is necessary to maintain the health of the periodontium (Lang & Löe 1972), this concept is not accepted today. However, a wide band of attached gingiva does offer certain advantages in the case of periodontal surgery, both therapeutically and esthetically.
Col—Interpapillary Saddle
Apical to the contact area between two teeth, the interdental gingiva assumes a concave form when viewed in labiolingual section. The concavity, the “col,” is thus located between the lingual and facial interdental papillae and is not visible clinically. Depending on the expanse of the contacting tooth surfaces, the col will be of varying depth and breadth. The epithelium covering the col consists of the marginal epithelia of the adjacent teeth (Cohen 1959, 1962; Schroeder 1992). The col is not keratinized. In the absence of contact between adjacent teeth, the keratinized gingiva courses uninterrupted from the facial to the oral aspect.
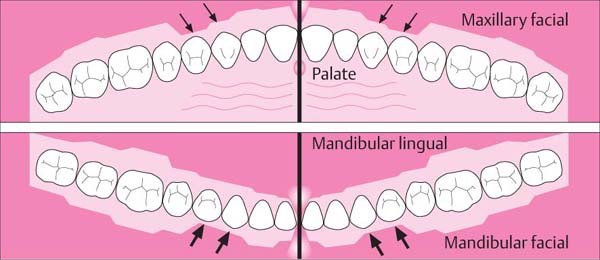
14 Mean Width of Attached Gingiva
• In the maxilla, the facial gingiva in the area of the incisors is wide, but narrow around the canines and first premolars. On the palatal aspect, the marginal gingiva blends without demarcation into the palatal mucosa.
• In the mandible, the lingual gingiva in the area of the incisors is narrow, but wide on the molars. On the facial, the gingiva around the canines and first premolars is narrow (arrow), but wide around the lateral incisors.
15 Variability of Gingival Width
Width of attached gingiva can vary dramatically. The three patients depicted here, all about the same age, exhibit gingival width varying from 1 to 10 mm in the mandibular anterior area.
Right: After staining the mucosa with iodine (Schiller or Lugol solution) the mucogingival line is easily visible because the non-keratinized alveolar mucosa is iodine-positive while the keratinized gingiva is not (cf. p. 161).
16 Col—Interpapillary Saddle
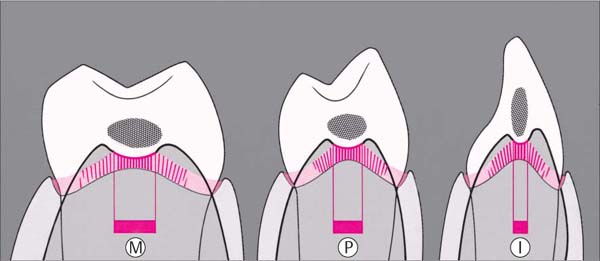
The col consists essentially of a connection between the junctional epithelia (JE; p. 10) of any two adjacent teeth. Tooth morphology, width of the tooth crowns and relative tooth position will determine the orofacial and the coronoapical extent of the contact surfaces (hatched) and their breadth (2–7 mm, red bar), as well as the depth (1–2 mm) of the interpapillary saddle.
| I | Incisor |
| P | Premolar |
| M | Molar |
Epithelial Attachment
Junctional Epithelium—Epithelial Attachment—Gingival Sulcus
The marginal gingiva attaches to the tooth surface by means of the junctional epithelium, an attachment that is continuously being renewed throughout life (Schroeder 1992).
Junctional Epithelium
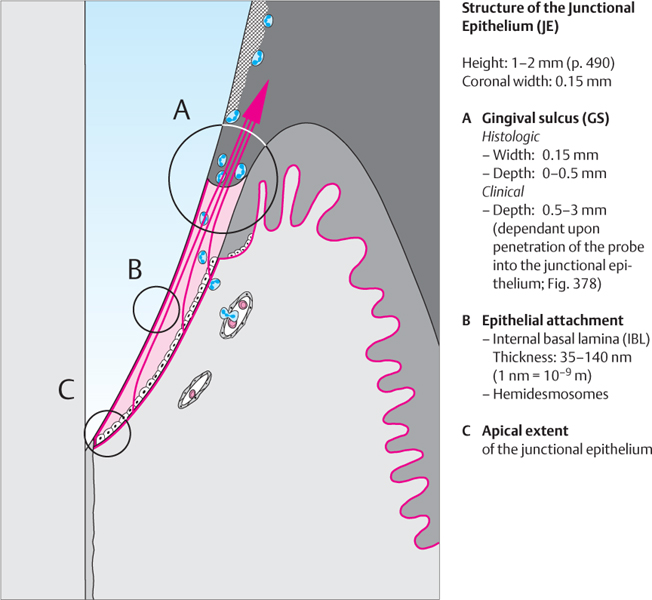
The junctional epithelium (JE) is approximately 1–2 mm in coronoapical dimension, and surrounds the neck of each tooth. At its apical extent, it consists of only a few cell layers; more coronally, it consists of 15–30 cell layers. Subjacent to the sulcus bottom, the JE is about 0.15 mm wide.
The junctional epithelium consists of two layers, the basal (mytotically active) and the suprabasal layer (daughter cells). It remains undifferentiated and does not keratinize. The basal cell layer interfaces with the connective tissue via hemidesmosomes and an external basal lamina. Healthy JE exhibits no rete ridges where it contacts the connective tissue. JE turnover rate is very high (4–6 days) compared to oral epithelium (6–12 days, Skougaard 1965; or up to 40 days, Williams et al. 1997).
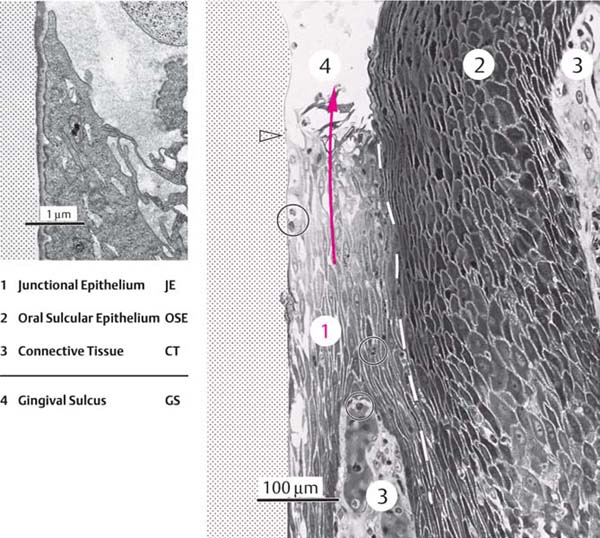
17 Junctional Epithelium and Gingiva in Orofacial Section
The gingiva consists of three tissues:
• Junctional epithelium
• Oral epithelium
• Lamina propria (connective tissue)
The junctional epithelium (JE) assumes a key role in maintenance of periodontal health: it produces the epithelial attachment and therefore creates the firm connection of soft tissue to the tooth surface. It is quite permeable, and thus serves as a pathway for diffusion of the metabolic products of plaquebacteria (toxins, chemotactic agents, antigens, etc.). There is also diffusion in the opposite direction, of host defense substances (serum exudates, antibodies, etc.). Even when the gingivae do not appear inflamed clinically, the JE is constantly trans-migrated by polymorphoneuclear leukocytes (PMNs) moving towards the sulcus (p. 55, Fig. 109). The red arrows depict the migration of daughter cells from the basal layer toward the gingival sulcus. The circled areas A-C are depicted in detail on page 11.
Epithelial Attachment
The epithelial attachment to the tooth is formed by the JE, and consists of an internal basal lamina (IBL) and hemidesmosomes. It provides the epithelial attachment between gingiva and tooth surface. This can be upon enamel, cementum or dentin in the same manner. The basal lamina and the hemidesmosomes of the epithelial attachment are structural analogs of their counterparts comprising the interface between epithelium and connective tissues.
All cells of the JE are in continual coronal migration, even those cells in immediate contact with the tooth surface. Such cells must continually dissolve and reestablish their hemidesmosomal attachments. Between the basal lamina and the tooth surface, a 0.5–1 μm thick “dental cuticle” is observed; this is possibly a serum precipitate or a secretion product of the junctional epithelial cells.
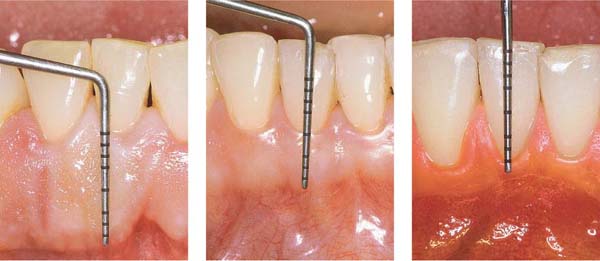
Gingival Sulcus
The sulcus is a narrow groove surrounding the tooth, about 0.5 mm deep. The bottom of the sulcus is made up of the most coronal cells of the junctional epithelium, which are sloughed (exfoliated) in rapid succession. One lateral wall of the sulcus is made up of the tooth structure, the other wall is the oral sulcular epithelium (OSE; Schroeder 1992).
18 Gingival Sulcus and Junctional Epithelium
The junctional epithelial cells (1) are oriented parallel to the tooth surface and are sharply demarcated (broken line) from the more deeply staining cells of the oral sulcular epithelium (2). All of the daughter cells that emanate from the entire 1–2 mm length of the basal layer of the junctional epithelium must transmigrate the exceptionally narrow (100–150 μm) sulcus bottom (red arrow). Note the polymorphonuclear leukocytes (circled), which emigrate from the venule plexus in the subepithelial connective tissue (3) without altering it in any way.
Left: In the enlargement, a portion of the most coronal JE cells (cf. empty black arrow in lower power view) is shown still manifesting hemidesmosomes and an internal basal lamina attached to the enamel surface.
Courtesy H. Schroeder
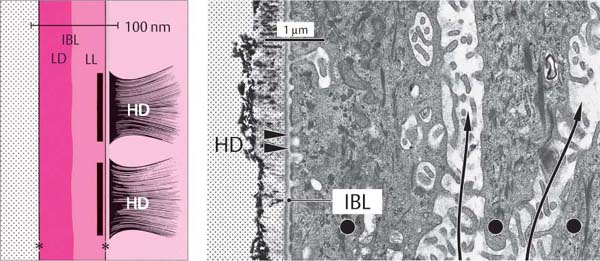
19 Internal Basal Lamina and Hemidesmosomes
Each JE cell adjacent to the tooth forms hemidesmosomes (HD) that enable these cells to attach to the internal basal lamina (IBL) and ultimately to the surface of the tooth. Remnants of enamel crystals are visible at the left. The long arrows indicate intercellular spaces between three JE cells (•).
Left: The basal lamina is comprised of two layers: the Lamina lucida (LL) and the Lamina densa (LD).
20 Apicalmost Portion of the Junctional Epithelium
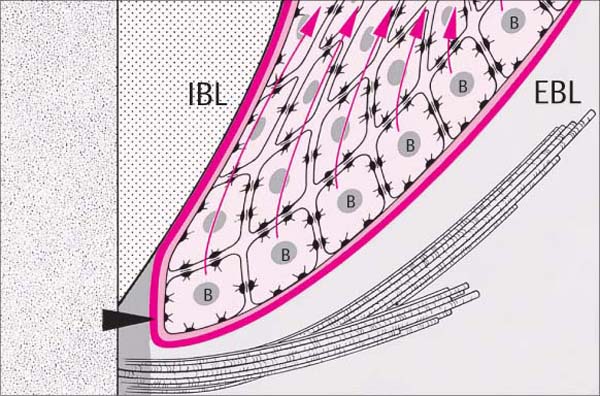
In a young, healthy patient, the JE ends apically at the cemento-enamel junction. Daughter cells of the cuboidal basal cells (B) migrate toward the sulcus (red arrows). If a JE cell comes into contact with the tooth surface, it establishes the attachment mechanism described above. The internal basal lamina (IBL) is continuous with the external basal lamina (EBL) around the apical extent of the JE (black arrowhead).
Connective Tissue Attachment
Gingival and Periodontal Fiber Apparatus
The fibrous connective tissue structures provide the attachment between teeth (via cementum) and their osseous alveoli, between teeth and gingiva, as well as between each tooth and its neighbor. These structures include:
• Gingival fiber groups
• Periodontal fiber groups (periodontal ligament)
Gingival Fiber Groups
In the supra-alveolar area, collagen fiber bundles course in various directions. These fibers give the gingiva its resiliency and resistance, and attach it onto the tooth surface subjacent to the epithelial attachment. The fibers also provide resistance to forces and stabilize the individual teeth into a closed segment (Fig. 22). The periosteogingival fibers are also a component of the gingival fiber complex. These connect the attached gingiva to the alveolar process.
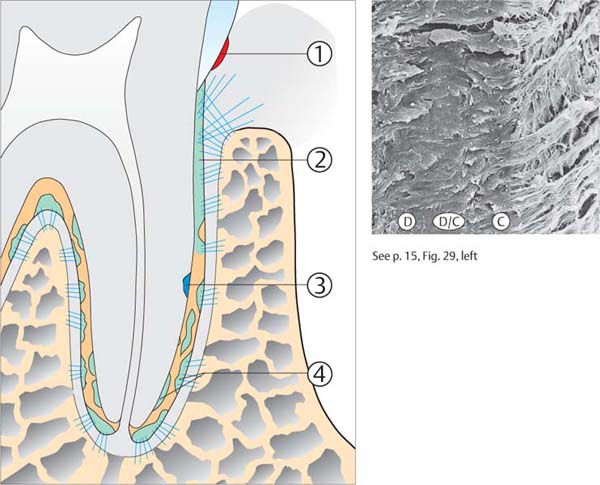
21 Localization and Orientation of Gingival and Periodontal Ligament Fiber Bundles (see also Fig. 22)
In the supra-alveolar region, within the free marginal gingiva and partially also the attached gingiva, the connective tissue compartment is composed primarily of collagen fiber bundles (A). These splay from the cementum of the root surface into the gingiva. Other fiber bundles course more or less horizontally within the gingiva and between the teeth, forming a complex architecture (Fig. 22). In addition to collagen fibers, one may also observe a small number of reticular (argyrophilic) fibers.
The periodontal ligament space (B) in adults is ca. 0.15–0.2 mm wide. About 60 % of the space is occupied by collagen fiber bundles. These fibers traverse from cementum to alveolar bone (C).
Right: Marginal gingiva. Fiber-rich connective tissue (A, blue), junctional epithelium and oral epithelium (reddish brown).
Histology courtesy N. Lang
Periodontal Fiber Groups, Periodontal Ligament
The periodontal ligament (PDL) occupies the space between the root surface and the alveolar bone surface. The PDL consists of connective tissue fibers, cells, vasculature, nerves and ground substance. An average of 28,000 fiber bundles insert into each square millimeter of root cementum!
The building block of a fiber bundle is the 40–70 nm thick collagen fibril. Many such fibrils in parallel arrangement make up a collagen fiber. Numerous fibers combine to form collagen fiber bundles. These collagen fiber bundles (Sharpey’s fibers) insert into the alveolar bone on one end and into cementum at the other (Feneis 1952). The most ubiquitous cells are fibroblasts, which appear as spindle-shaped cells with oval nuclei and numerous cytoplasmic processes of varying lengths. These cells are responsible for the synthesis and break-down of collagen (“turnover”). Cells responsible for the hard tissues are the cementoblasts and osteoblasts. Osteoclastic cells are only observed during phases of active bone resorption. Near the cementum layer, within the PDL space, one often observes string-like arrangements of epithelial rest cells of Malassez.
The periodontal ligament tissues are highly vascularized (p. 18) and innervated (p. 19).
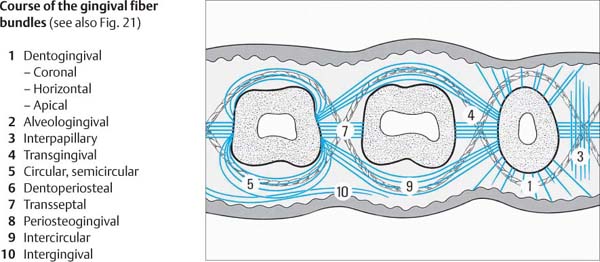
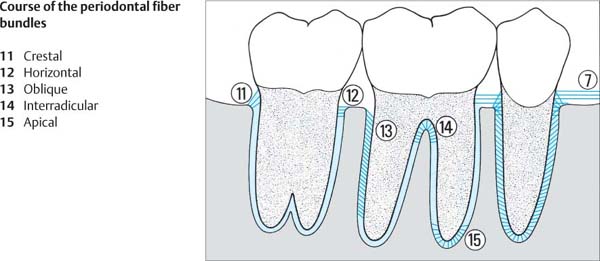
22 Fiber Apparatus in Horizontal Section
The course of the most important supracrestal (gingival) fiber bundles is depicted. The connections between teeth and gingiva as well as between individual teeth are clearly shown.
23 Fiber Bundles Viewed in Mesiodistal Section
In the interdental area, the transseptal fiber bundles (7) extend supragingivally from one tooth to its neighbor. The fibers stabilize the arch in its mesiodistal dimension (courtesy N. Lang).
Left: The basic element of the fiber bundle is the collagen fibril (cut in cross and longitudinal sections); such fibrils are secreted by fibroblasts, and exhibit a regular 64 nm banding pattern (compare the wavelength of blue light, 400 nm). TEM courtesy H. Schroeder
Periodontal Fibers
24 Fiber Apparatus in Mesiodistal Section
The anchoring of a tooth in the alveolar bone is accomplished via the dentoalveolar fibers of the periodontal ligament (PDL). Occlusal forces are absorbed primarily by the oblique fibers, which course from bone to cementum (13). The remaining fiber bundles (11, 12, 14, 15) counteract tipping and rotating forces.
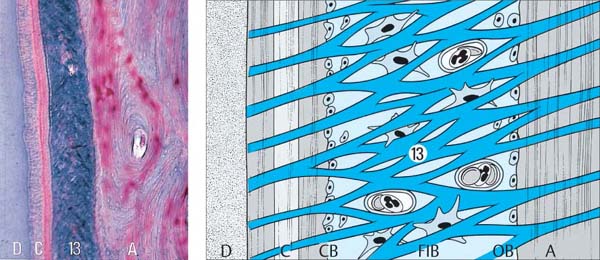
25 Periodontal Ligament—Details
Collagen fiber bundles (13) are intertwined. Osteoblasts (OB) line the surface of the bone; cementoblasts (CB) line the cementum surface; numerous fibroblasts (FIB) occupy the PDL space.
Left: The histologic section (Azan, x50) depicts the fiber-rich periodontal ligament (13) and its relationship to cementum (C) and bone (A). D = Dentin.
Histology courtesy N. Lang
Root Cementum
Types of Cementum
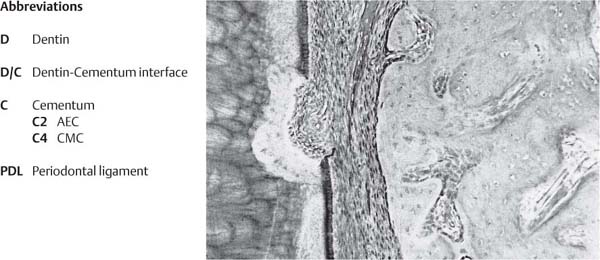
From a purely anatomic standpoint, root cementum is part of the tooth, but also part of the periodontium. Four types of cementum have been identified (Bosshardt & Schroeder 1991, 1992; Bosshardt & Selvig 1997):
| 1 Acellular, afibrillar cementum | (AAC) |
| 2 Acellular, extrinsic-fiber cementum | (AEC) |
| 3 Cellular intrinsic-fiber cementum | (CIC) |
| 4 Cellular mixed fiber cementum | (CMC) |
AEC and CMC are the most important types of cementum.
Cementum-forming Cells
Fibroblasts and cementoblasts collaborate in the formation of cementum. Periodontal ligament fibroblasts secrete acellular extrinsic cementum. Cementoblasts secrete cellular intrinsic cementum, and a portion of the cellular mixed fiber cementum, and probably also acellular afibrillar cementum. Cementocytes evolve from the cementoblasts, which become entrapped in cementum during cementogenesis. As a result, cementocytes are observed within cellular mixed fiber cementum and frequently in cellular intrinsic cementum (see also Cementum Formation and Healing, p. 206).
26 Types of Cementum—Structure, Localization and Development
1 Acellular, Afibrillar Cementum (AAC; red) AAC is formed at the most cervical enamel border following completion of pre-eruptive enamel maturation, and sometimes also during tooth eruption. It is probably secreted by cementoblasts.
2 Acellular, Extrinsic-fiber Cementum (AEC; green) AEC forms both pre- and post-eruptively. It is secreted by fibroblasts. On the apical portions of the root, it comprises a portion of the mixed-fiber cementum.
3 Cellular, Intrinsic-fiber Cementum (CIC; blue) CIC is formed both pre- and post-eruptively. It is synthesized by cementoblasts, but does not contain extrinsic Sharpey’s fibers.
4 Cellular, Mixed-fiber Cementum (CMC; orange/green) CMC is formed by both cementoblasts and fibroblasts; it is a combination of cellular intrinsic-fiber cementum and acellular extrinsic-fiber cementum.
Acellular Extrinsic Cementum (AEC)
The AEC is primarily responsible for the anchorage of the tooth in the alveolus. It is found in the cervical third of all deciduous and permanent teeth. The AEC consists of tightly packed and splaying fiber bundles (Sharpey’s fibers), which are embedded in the calcified cementum.
The collagenous structures of cementum and dentin intertwine with each other during root formation and before calcification. This phenomenon explains the tight connection between these two hard tissues.
AEC is the type of cementum that is desired following regenerative periodontal surgical procedures.
Cellular Mixed-fiber Cementum (CMC)
The CMC is also of importance for the anchorage of the tooth in its alveolus. But it is only the acellular extrinsic-fiber cementum portion (AEC) within the mixed cementum, into which the Sharpey’s fibers secreted by fibroblasts insert and therefore affix the tooth. CMC is layered vertically but also horizontally to the root surface. The portions secreted by cementoblasts contains high numbers of cementocytes (Fig. 30, left). The CMC is also tightly affixed to the dentin because of the intertwining of the collagen fiber bundles during tooth formation. The CMC “grows” faster than AEC.
27 Acellular, Afibrillar Cementum (AAC)
AAC is observed only at the cervical region of the tooth, at the cementoenamel junction. It appears as small “islands” upon the enamel and sometimes also on the marginal areas of the root. AAC is formed during tooth eruption, when the reduced enamel epithelium partially dissolves as the enamel surface comes into contact with connective tissue.
28 Acellular, Extrinsic-fiber Cementum (AEC)
AEC is localized to the coronal third of the root (C), and exhibits a horizontal fiber structure (blue). Variations in the directionality may occur when the tooth encounters positional changes during cementum formation.
Left and p. 14: Note the intense intertwining and connections between dentin (D) and cementum (C), as well as of cementum and periodontal ligament (PDL).
29 Cellular, Intrinsic-fiber Cementum (CIC)
CIC is usually a component of mixed-fiber cementum. It is localized to the middle, apical and furcal regions of the roots and usually contains embedded cementocytes.
As shown in this figure, CIC is also “reparative” cementum, i. e., it can repair resorption areas and root fractures.
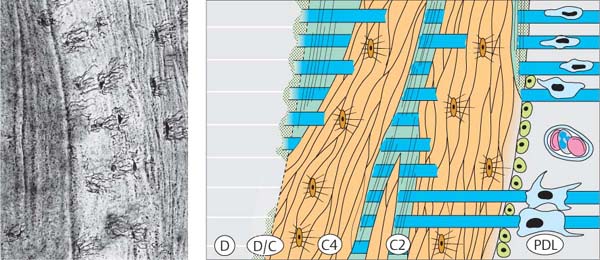
30 Cellular, Mixed-fiber Cementum (CMC)
CMC is found in the apical portion of the root and in the furcation region. It represents a mixture of acellular extrinsic-fiber cementum (C2) and cellular intrinsic-fiber cementum (C4).
Left: “Medusa”-like cementocytes in the apical region of a multi-rooted tooth.
Adapted from D. Bosshardt, H. Schroeder
Osseous Support Apparatus
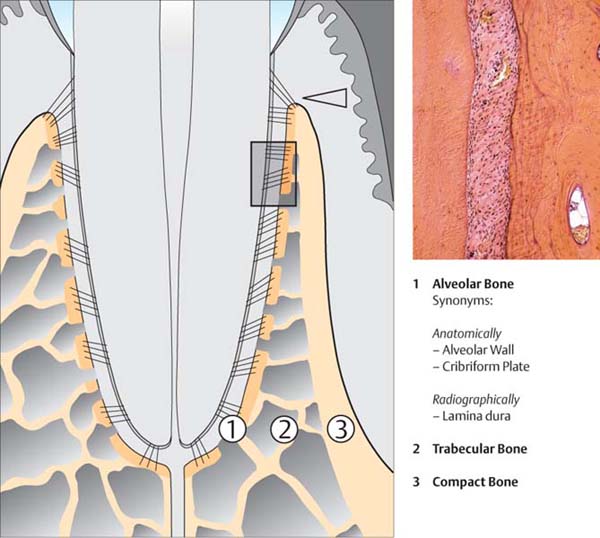
Alveolar Process—Alveolar Bone
The alveolar processes of the maxilla and the mandible are tooth-dependent structures. They develop with the formation of and during the eruption of the teeth, and they atrophy for the most part after tooth loss. Three structures of the alveolar process may be discriminated:
• Alveolar bone proper
• Trabecular bone
• Compact bone
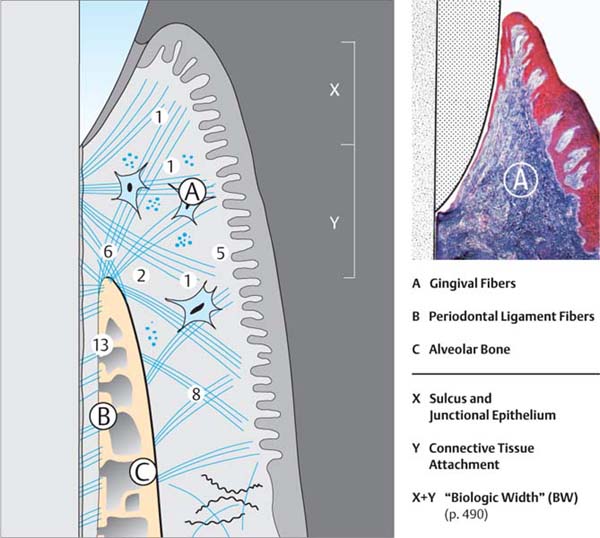
Compact bone covers and contains the alveolar processes. At the entrance to the alveoli, the alveolar crest, it blends into the cribriform plate, the alveolar bone proper, which forms the alveolar wall and is approximately 0.1–0.4 mm thick. It is perforated with numerous small canals (Volkmann canals) through which vessels as well as nerve fibers enter into and exit the periodontal ligament space. The trabecular bone occupies the space between compact bone and alveolar bone proper. The distance between the marginal gingiva and the alveolar crest is referred to as the “biologic width” of 2–3 mm (Gargiulo et al. 1961, see p. 490).
31 Osseous Support Apparatus
The tooth-supporting alveolar process consists of the alveolar bone (1), trabecular bone (2), and compact bone (3). Alveolar bone and compact bone join at the margin to form the alveolar crestal bone (arrow). In this region, the alveolar process is often extremely thin, especially on the facial aspect, and unsupported by trabecular bone (Fig. 36).
Right: Histologic section (HE, x10) through the periodontium (the location is indicated by the rectangle superimposed in the main figure). On the right side of the picture, the alveolar bone with its osteons and a Haversian canal is clearly visible. Bundle bone has been deposited adjacent to the structures on the periodontal aspect.
The periodontal ligament is cell-rich, and exhibits a thin layer of cementum-forming fibroblasts along the acellular, extrinsic-fiber cementum (left).
Histology courtesy H. Schroeder
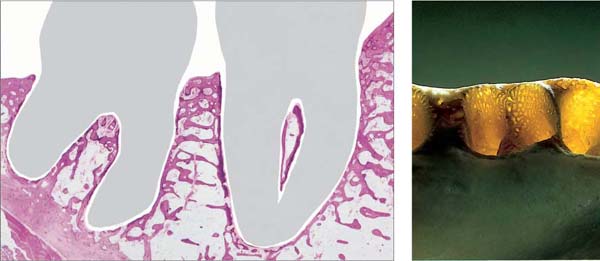
32 Mandibular Alveolar Process in Sagittal Section
In this histologic section (H and E, x1) the elegant structure of the trabecular bone and the more or less large marrow spaces are visible. The alveolar bone proper is depicted only as a very thin, often partially broken line.
Right: In this transilluminated bone preparation, it becomes clear that the alveolar bone is perforated by numerous small holes, as in a sieve (cribriform plate).
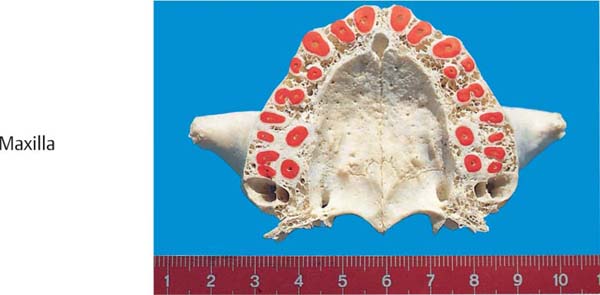
33 Maxillary Alveolar Process in Horizontal Section
The section is through the alveolar process and tooth roots at about the midpoint. With the exception of the molar areas, the bone is thicker on the oral surface than on the facial. The trabecular bone is variably thick. Clearly visible is the varying mass of the bony interdental and interradicular septa, as well as the varying shape of the root cross sections.
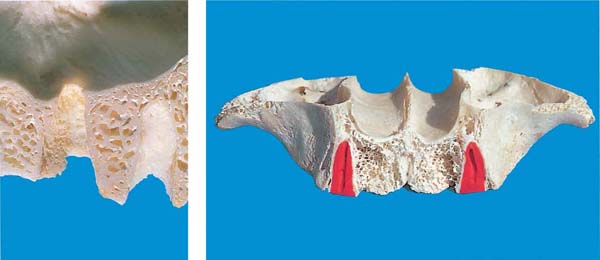
34 Maxilla—Frontal Section
This section was cut through the plane of the canine, and it shows the relationship of the root to the nasal sinus. Clearly visible is the very thin bony layer on the facial surface of the root (Fig. 36).
Left: The sagittal section shows how the root tips of premolars and molars sometimes extend into the maxillary sinus. The alveolar bone may actually border directly on the sinus mucosa.
Mandibular Bone
35 Mandibular Alveolar Process in Horizontal Section
The section represents a cut approximately halfway down the tooth root. In contrast to the maxilla, the orofacial width of the mandibular alveolar process is considerably less. All roots exhibit an “hourglass” profile (proximal concavities).
36 Orofacial Sections through the Mandible
From right to left, an incisor, a canine, a premolar and two molars were sectioned. Impressive is the thin, tapered bony lamella on the facial aspect; it is impossible to distinguish between compact bone and alveolar bone proper.
Left: Sagittal section through the alveolar process and alveoli. Especially in the molar area, the alveolar bone is traversed by numerous Volkmann canals.
Blood Supply of the Periodontium
All periodontal tissues, but especially the periodontal ligament, have a copious blood supply even in the healthy state. This is due not only to the high metabolism of this cell- and fiber-rich tissue, but also to the peculiar mechanical/functional demands on the periodontium. Occlusal forces are resisted not only by the periodontal ligament and the alveolar process, but also by means of the tissue fluid and its transfer within the periodontal ligament space (hydraulic pressure distribution, dampening).
The most important afferent vessels for the alveolar process and the periodontium are:
|
• In the maxilla, the anterior and posterior alveolar arteries, the infraorbital artery and the palatine artery • In the mandible, the mandibular artery, the sublingual artery, the mental artery, and the buccal and facial arteries. |
Lymph vessels follow for the most part the blood vascular tree.
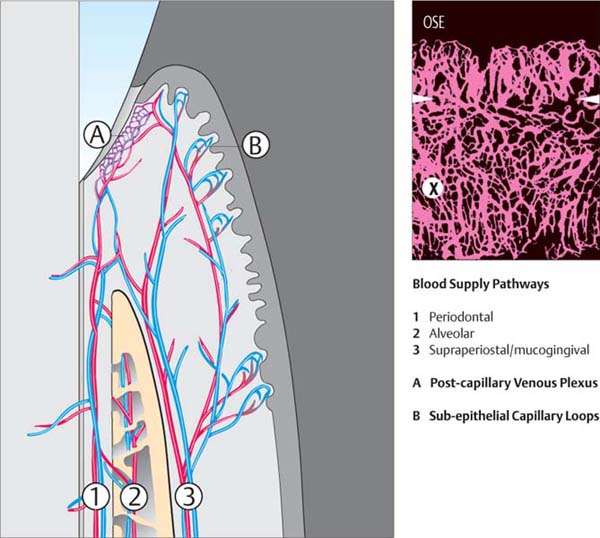
37 Diagram of Periodontal Blood Supply
The periodontal ligament (1), the alveolar process (2) and the gingiva (3) are supplied by three vascular sources. These vessels exhibit frequent anastomoses.
Within the periodontal ligament, the vascular network is especially dense. Adjacent to the junctional epithelium, these vessels splay into a very dense vascular system, the capillary-venule plexus (A). This plexus is of great significance for host defense against infection (p. 55).
The oral epithelium contacts the subjacent connective tissue through a series of rete ridges. Each rete ridge of connective tissue contains capillary loops (B).
Right: In this section beneath the JE, a dense vascular plexus (X) is observed even in health. Above the white arrows, one observes the most marginal vascular loops in the area of the adjacent, nonkeratinized oral sulcular epithelium (OSE).
Courtesy J. Egelberg
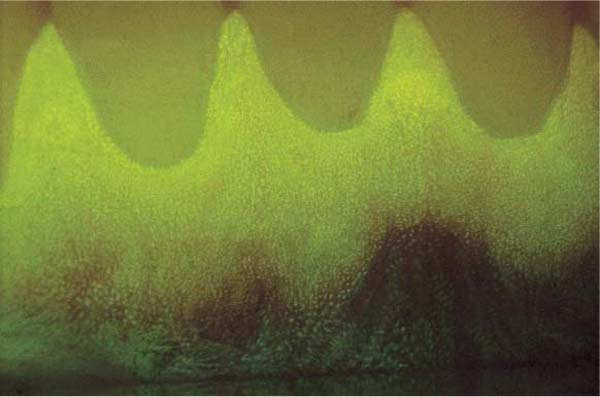
38 Fluorescence Angiography—Vascular Loops Subjacent to the Oral Epithelium
Following intravenous injection of 2 ml of sodium fluorescein solution (20 %), the vessels (capillaries) subjacent to the oral epithelium can be rendered visible by UV light.
Some small vascular loops are visible in the connective tissue rete pegs (B in Fig. 37).
Courtesy W. Mörmann
Innervation of the Periodontium
The sensory innervation of the maxilla occurs via the second branch of the trigeminal nerve, and that of the mandible via the third branch. The following description of the neural distribution within the periodontal structures is based upon investigations by Byers (1985), Linden et al. (1994) and Byers & Takeyasu (1997).
The periodontium, especially the gingiva and periodontal ligament, contains “Ruffini-like” mechanoreceptors and nociceptive nerve fibers, in addition to the ubiquitous branches of the sympathetic nervous system.
The functions of these innervations are coordinated with those of the dental pulp and the dentin. The stimulus threshold of the mechanoreceptors, which react to tactile (pressure) stimulus, as well as to the stretching of the periodontal ligament fibers, is very low. In contrast, the pain-sensing nociceptive nerve endings have a relatively high threshold. It is via these two separate afferent systems that “information” about jaw position, tooth movements, speech, tooth contact during swallowing and chewing, minor positional alterations (physiologic tooth mobility), pain during unphysiologic loading, as well as injuries are transmitted. In this way, various mechanoreceptors transmit “conscious reactions” via trigeminal ganglia to the sensory nucleus of the trigeminal in the central nervous system, while unconscious reflexes transmit to mesencephalic sensory neurons. These various receptors are localized in varying regions of the periodontal structures: At the level of the middle of the root, one finds more receptors for up-take of “conscious reactions,” whereas in the apical region there are more receptors for the unconscious reflexes whose signals transmit to the mesencephalic sensory neurons.
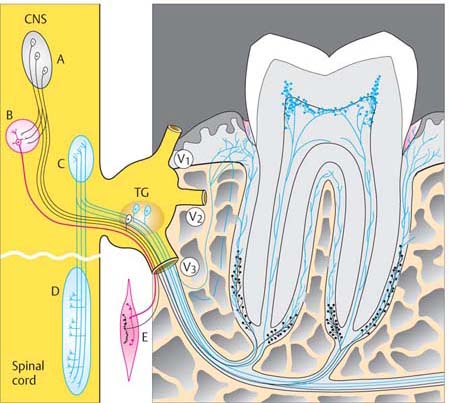
39 Innervation of a Mandibular Molar
Innervation of gingival and periodontal structures is via the mandibular nerve, the third branch of the trigeminal nerve.
Modified from M. Byers
| A | Mesencephalic sensory neurons of the trigeminal nerve |
| B | Motor nucleus of the trigeminal |
| C | Sensory nucleus of the trigeminal |
| D | Spinal sensory trigeminal nucleus |
| E | Fibers of the masticatory musculature |
| TG | Trigeminal ganglion (Gasserian ganglion) with its three branches:
V1 Ophthalmic V2 Maxillary V3 Mandibular |
| CNS | Central Nervous System |
The junctional epithelium as well as the epithelia of the free and attached gingiva, neither of which are vascularized, are served by a dense network of nociceptive and tactile nerve endings. The same is true for the subepithelial, supracrestal gingival connective tissue.
Somatosensory perception in certain gingival diseases (e. g., ulcerative gingivoperiodontitis), as well as pressure and pain sensation during probing of the healthy gingival sulcus or periodontal pockets are the clinical manifestations of the innervation of gingival tissues.
The Coordinated Functions of the Periodontal Structures
Turnover—Adaptation—Defense—Healing
Within the healthy periodontium, there is a constant turnover of all tissues, except cementum. The term tissue homeostasis was coined to characterize the process by which the composition of the various structures, i. e., the balance of their volumes, their integrity vis-à-vis each other, and their mutually interwoven functions (Williams et al. 1997). The apposition and/or resorption of the various tissues can vary even in the healthy condition, depending upon several factors; for example, the periodontal tissues undergo a process known as adaptation, or in cases of reduced occlusal loading (afunction, hypofunction) or increased loading (hyperfunction, parafunction). This concept is not limited only to an adaption to masticatory forces, but is broader in the sense of all “insults” that the periodontal tissues may encounter, including the always present—although in very differing degrees—infection.
Host defense against all “attacks” refers primarily to the immune system (p. 41), but also to the healthy tissue. Disease (periodontitis) will ensue if the demands upon the tissues are larger than their capability to reactively adapt.
The adaptability of the tissues, i. e., their ability to vary the rate of turnover in response to various mediators (e. g., cytokines) also plays an important role in healing, for example following injury or after mechanical periodontal therapy (scaling and root planing—S/RP).
Primary Functions of the Periodontal Tissues
Epithelium
The epithelium of the attached gingiva (OE, oral epithelium) is referred to as masticatory mucosa. Like the palatal mucosa, it is keratinized. Keratinization is a mechanism for protection against all mechanical challenges, but also against thermal, chemical and infectious attack.
The turnover rate of the gingival epithelium has been variously reported between 6 (Schroeder 1992) and 40 days (Williams et al. 1997). Probably it is influenced by “chalones” (substances which inhibit mitosis) on the one hand and by cytokines (Fig. 95), e. g., epidermal growth factor (EGF) and transforming growth factor (TGF-b) on the other.
The junctional epithelium exhibits a faster turnover rate than gingival epithelium. Cell division occurs in the basal cell layer. All of the daughter cells migrate in the direction of the gingival sulcus, where they are rapidly sloughed.
Through this constant flow of junctional epithelial cells, sulcus fluid, and active migration of granulocytes (PMN), the sulcus is effectively cleared of invading bacteria and their metabolic products. In addition to immunocompetent cells, it is also primarily the dynamics of the resident tissue cells and the coronally streaming flow of tissue fluid that is responsible for the prevention of infection and therefore also for the maintenance of health of the marginal periodontal structures.
Gingival Connective Tissue
The circumstance for the periodontal connective tissue is similar to that of the epithelial structures. It has a turnover rate of only a few days, orchestrated by cytokines and growth factors (platelet-derived growth factor/PDGF; fibroblast growth factor/FGF, among others). In this matrix, it is the fibroblasts that are responsible for the synthesis and breakdown of collagen matrix. The matrix metalloproteinases (Fig. 102) that are responsible for the breakdown of collagen depend upon divalent cations (e. g., Zn+2). The balance between synthesis and breakdown can be “shifted” to a certain degree toward synthesis in the presence of pathogenic influences. However, if the pathogenic insult is too great, the process of increased resorption (or reduced synthesis?) can occur and lead ultimately to tissue destruction.
Periodontal Bone
Bone synthesis and resorption, especially the bone loss characteristic of periodontitis, is covered in detail in the chapter “Etiology and Pathogenesis” (pp. 60–61).
Cementum
In contrast to epithelium, connective tissue and bone, cementum is not subject to continuous turnover. Throughout life, cementum tends to increase in thickness due to apposition. Local resorption observed as resorption lacunae may result from trauma, orthodontic forces, or they may be idiopathic. Such defects are often “repaired” by synthesis of cellular intrinsic-fiber cementum.
Summary
The healthy periodontal structures have the capacity to mount a certain defense potential even before the actual immune response is mounted; the latter, of course, also introduces certain destructive elements into the milieu.
Etiology and Pathogenesis
The most common diseases of the tooth-supporting apparatus are plaque-induced, usually chronic, inflammatory alterations in the gingiva and the subjacent periodontal structures. Gingivitis may persist for many years without progressing to periodontitis. With good oral hygiene and effective professional removal of plaque and calculus, gingivitis is completely reversible. Periodontitis usually develops out of a more or less pronounced gingivitis. Periodontitis is only partially reversible (see periodontal healing, p. 205; regenerative therapies, p. 323).
The reasons why gingivitis develops into periodontitis (or does not) are still incompletely understood. As with all infections, it appears that the proliferation of pathogenic microorganisms, their toxic potency, their capacities to invade tissues, and above all the individual host response to such infection are the determining factors (p. 55; Kornman et al. 1997, Page & Kornman 1997, Salvi et al. 1997).
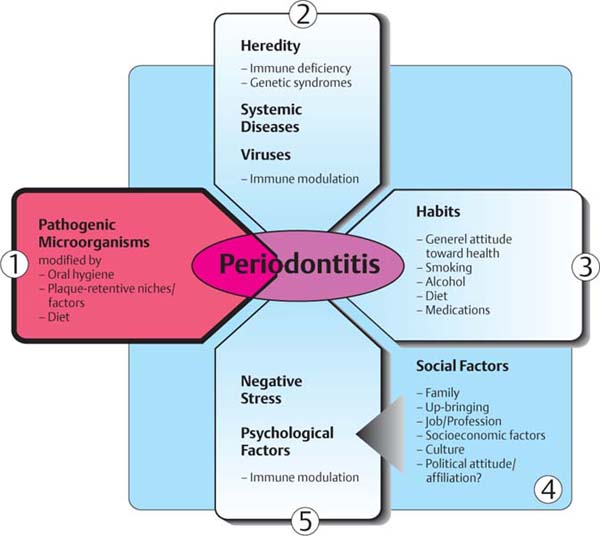
40 Bacterial Attack vs. Defense Reactions by the Host
The virulence of bacteria, their capability to invade into tissues, their number and composition, as well as their metabolic products elicit reactions by the host (immune response). Whether periodontal disease will occur, and its severity, depends only partially upon the bacterial insult. Transition from gingivitis to periodontitis involves factors such as host response, risk factors, and local factors.
An absolutely plaque-free condition in the oral cavity, with prevention of any biofilm formation on tooth surfaces is unachievable, and illusion, and probably even unphysiologic. Nevertheless, gingival and periodontal health can be maintained if the accumulation of plaque is small and if the biofilm contains only weakly virulent organisms (gram-positive, facultative anaerobes), and if an effective host response is mounted.
If the bacterial flora takes on periodontally pathogenic characteristics (e. g., certain gram-negative microorganisms), the result will be inflammation and specific immunologic responses; these responses may represent not only defense mechanisms, but—especially in long-term chronic infection—also destructive potential (cytotoxic, immunopathologic; p. 34).
Inflammation-inducing products of bacteria include enzymes, antigens, toxins, and “signal” substances that activate macrophages and T-cells (Birkedal-Hansen 1998). It is likely that bacterial enzymes, other metabolic products and toxins can directly elicit injury to the periodontal tissues even without immediate host response (inflammation). Bacterial products including hyaluronidase, chondroitin sulfatase, proteolytic enzymes, as well as cytotoxins in the form of organic acids, ammonia, hydrogen sulfide and endotoxins (e. g., lipopolysaccharides, LPS) have been demonstrated in periodontal tissues.
Periodontitis—A Multifactorial Disease
In recent years, the conceptual view concerning the etiology of periodontitis has evolved. Early on, it was the bacteria that were viewed as the determining factor. Certain pathogenic microorganisms were shown to be associated with various forms of periodontal disease, as well as the speed of progression. However, the existence and distribution of pathogenic bacteria did not always correlate with the inception and clinical progression of periodontitis. Furthermore, it was demonstrated that the presence of pathogenic bacteria in a periodontal pocket is not necessarily the cause of that pocket; rather, it seemed much more important that the pocket milieu presents a favorable environment for the existence and proliferation of pathogenic organisms. The stage would then be set—like a vicious cycle—for the progression of the disease processes (Mombelli et al. 1991).
Nevertheless, the old adage “no bacteria = no periodontitis” still holds true, but on the other hand it is also a fact that bacteria, including periodontopathic bacteria, do not without exception cause periodontitis.
41 Etiology of Periodontitis—Interaction between Dental Plaque and the Host
Bacteria
1 The primary etiologic factor for the existence of periodontitis is pathogenic microorganisms within the subgingival biofilm.
Host
2 The genetically determined non-specific and specific immune responses, as well as systemic syndromes and diseases influence the existence and the clinical course of periodontitis.
3 “Habits” and the patient’s own approach to general health will influence plaque formation and host immune response, both systemically and particularly with regard to oral health.
4 Social circumstances influence the systemic and psychic well being of the patient. Problems in the socioeconomic arena lead to negative stress.
5 Psychic burdens and stress influence the immune status.
In addition to specific microorganisms, diverse host factors are critical for the development of periodontitis from a preexisting gingivitis (cf. Fig. 41, modified from Clarke & Hirsch 1995). Such factors include the immune responses triggered by pathogens, and these are well understood today. Such defense reactions may be disproportional to the insult, resulting in immunopathologic tissue injury.
Recently, however, in addition to the genetically determined immune reactions, a great number of other individual risk factors have been identified, which may be responsible for the initiation and the degree of severity of the clinical course of periodontitis (p. 51).
Of the risk factors listed in Figures 41 and 104, only a few are capable of damaging the periodontium directly (e. g., smoking); of much greater importance is the influence of such factors on the patient’s own immune system. The delicate balance between “attack/destruction” (bacteria) and defense (host response) is disturbed. It is only logical to assume that the most severe, early-onset and aggressive forms of periodontitis will occur when particularly virulent bacteria are present in a weak (immunodeficient) host.
Microbiology
Bacteria are present throughout life in a myriad of sites on and in the human body. The bacteria may be beneficial for the host, or of no consequence (commensal), or injurious. In the oral cavity, over 530 different species of microorganisms have so far been identified; fortunately, for the most part these organisms remain in ecological balance and do not cause disease. Certain facultatively pathogenic (“opportunistic”) bacteria are occasionally observed in high numbers, e. g., in cases of disease (periodontitis, mucosal infection). It remains unclear whether these bacteria alone represent the cause of the diseases, or whether they simply find favorable living conditions in the disease milieu. Non-specific supragingival plaque (mixed flora) will elicit gingivitis within ca. seven days. If the plaque is removed, gingivitis regresses in a short period of time (“reversibility”). On the other hand, for the various forms of periodontitis, especially the aggressive, rapidly-progressing forms, specific bacteria are associated.
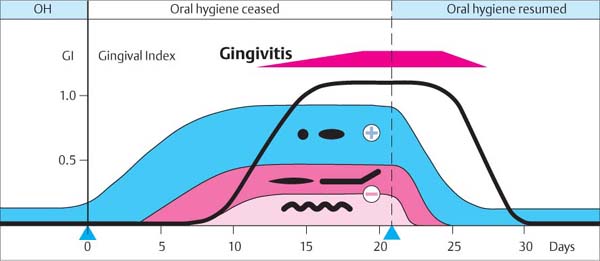
42 Experimental Gingivitis
In 1965, Löe et al. published the classic experimental proof of the bacterial etiology of gingivitis. In plaque- and inflammation-free subjects, plaque begins to accumulate if all oral hygiene is ceased. For the first few days, this plaque is composed of gram-positive cocci and rods, then later of filamentous organisms and finally spirochetes (gram-negative). Within a few days, a mild gingivitis ensues. If the plaque is removed, the gingiva quickly returns to a state of health.
43 Healthy Gingiva and Initial Gingivitis
Left: “Almost” clean central incisors. An extremely thin biofilm is compatible with clinically healthy gingiva. A few bacteria may even serve to maintain the “memory” of the immune system (p. 55).
Right: Plaque accumulation following seven days without oral hygiene. Gingivitis has developed. The proliferation and relative increase in numbers of gram-negative microorganisms are responsible for the gingivitis.
Biofilm—Plaque Formation on Tooth and Root Surfaces
The oropharynx is an open ecosystem wherein bacteria are always present; bacteria attempt to colonize in all favorable locations. Most bacteria, however, can only persist after the formation of a biofilm upon desquamation-free surfaces, i. e., hard substances (tooth and root surfaces, restorative materials, implants, prostheses etc.). In the presence of healthy dental and gingival relationships, there is a balance between the additive and retentive mechanisms of biofilms vis-à-vis the abrasive forces that tend to reduce biofilm formation, e. g., self-cleansing by the cheeks and tongue, diet and mechanical oral hygiene measures.
The existence of a biofilm results within a matter of hours or days, in the phases described below (Darveau et al. 1997, Descouts & Aronsson 1999, Costerton et al. 1999).
The establishment and stabilization of bacteria within a biofilm are important not only for the etiology of periodontitis, but also for adjunctive systemic and topical medicinal treatment for periodontitis (p. 287): Biofilm bacteria imbedded within a matrix of extracellular polysaccharides are more than 1,000 times less sensitive to antimicrobials (e. g., antibiotics) than free-floating (“planktonic”) bacteria.
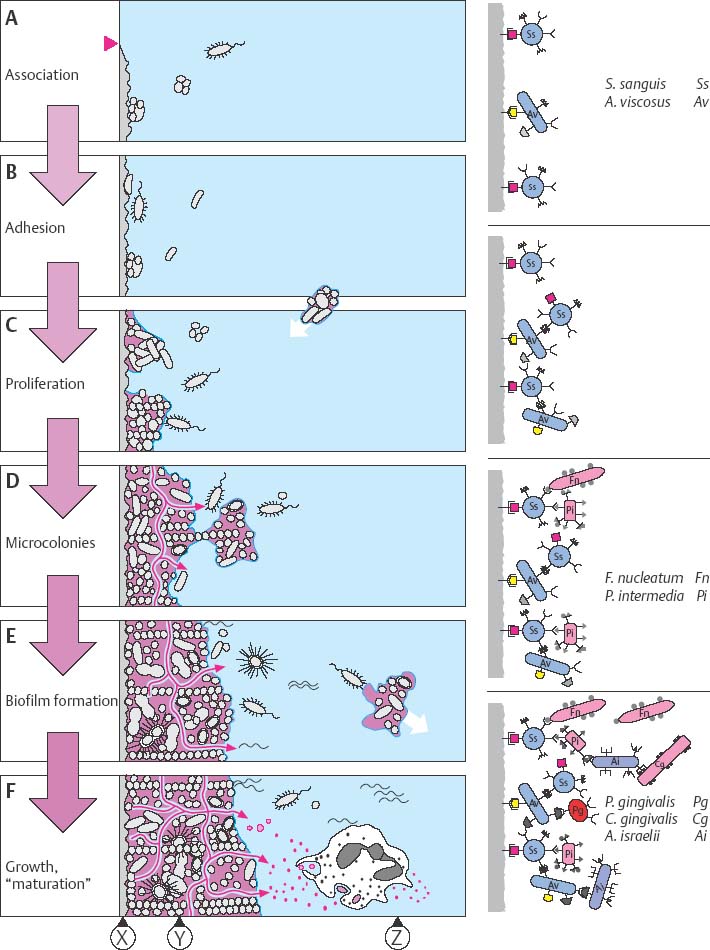
44 Dental Plaque—Development
Within minutes after completely cleansing the tooth surface, a pellicle forms from proteins and glycoproteins in saliva.
A Association: Through purely physical forces, bacteria associate loosely with the pellicle.
B Adhesion: Because they possess special surface molecules (adhesins) that bind to pellicle receptors, some bacteria become the “primary colonizers,” particularly streptococci and actinomyces. Subsequently, other microorganisms adhere to the primary colonizers.
C Bacterial proliferation ensues.
D Microcolonies are formed. Many streptococci secrete protective extracellular polysaccharides (e. g., dextrans, levans).
E Biofilm (“attached plaque”): Microcolonies form complex groups with metabolic advantages for the constituents.
F Plaque growth—maturation: The biofilm is characterized by a primitive “circulatory system.” The plaque begins to “behave” as a complex organism! Anaerobic organisms increase. Metabolic products and evulsed cell wall constituents (e. g., lipopolysaccharides, vesicles) serve to activate the host immune response (p. 38). Bacteria within the biofilm are protected from phagocytic cells (PMN) and against exogenous bacteriocidal agents.
| X | Pellicle |
| Y | Biofilm—“Attached Plaque” |
| Z | Planktonic Phase |
Supragingival Plague
… and its initial subgingival expansion
The first bacteria that accumulate supragingivally on the tooth surface are mostly gram-positive (Streptococcus sp, Actinomyces sp.). In the course of the following days, gram-negative cocci as well as gram-positive and gram-negative rods and the first filamentous forms begin to colonize (Listgarten et al. 1975, Listgarten 1976). By means of a variety of metabolic products, the bacterial flora provoke the tissue to an increase in exudation and to migration of PMN leukocytes into the sulcus (“leukocyte walls” against the bacteria).
The increase in PMN diapedesis and the flow of sulcus fluid lead to initial disintegration of the junctional epithelium. This makes it possible for bacteria to more easily invade between the tooth and the junctional epithelium, and invade the subgingival area (gingivitis, gingival pocket formation). In the total absence of oral hygiene, plaque formation and an initial host defensive response within gingival tissue occur. With optimum—including interdental—oral hygiene, the formation of biofilm is repeatedly disrupted and gingival health is maintained.
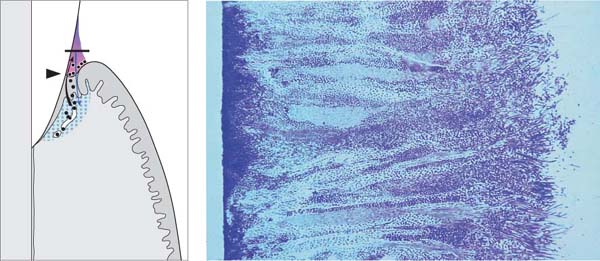
45 One-week-old Plaque—Interactions
Note the thick zone of early colonizers on the enamel surface and the column-like structures that result from rapid proliferation of streptococci. On the plaque surface, one observes rods and filaments.
Left: Interaction between host and plaque. Chemotactically regulated immigration of polymorphonuclear granulocytes (PMN, arrow). The black horizontal line indicates the level from which this sample of plaque was taken.
46 Three-week-old Plaque
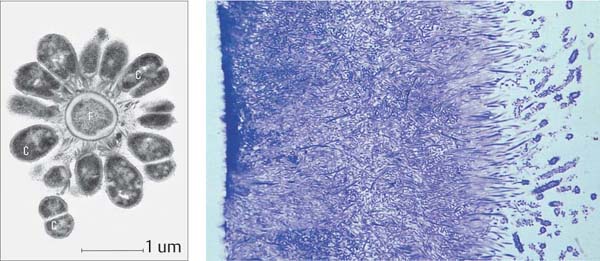
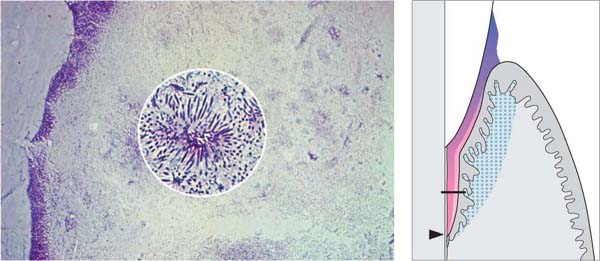
The composition of the supragingival plaque has changed markedly. Filamentous organisms now predominate. Conspicuous forms resembling “corn cobs” are observed at the plaque surface.
Left: In this transmission electron photomicrograph, the structure of such a “corn cob” is revealed. At the center is a gram-negative filamentous organism (F), surrounded by gram-positive cocci (C).
Histology and TEM courtesy M. Listgarten
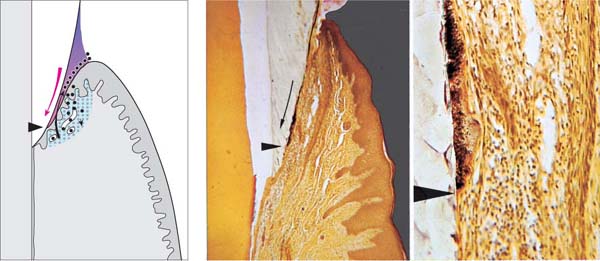
47 Expansion of Supragingival Plaque—Gingival Pocket
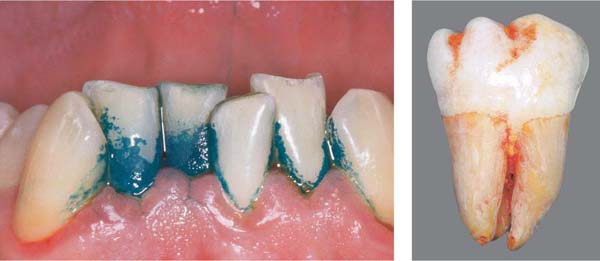
Middle and Right: Weakening of the epithelial attachment to the tooth permits apical migration of gram-positive plaque bacteria in a thin layer between the tooth and the junctional epithelium (thin arrow). Gram-negative bacteria colonize subsequently, and a gingival pocket forms (Fig. 150). Histology courtesy G. Cimasoni
Left: Schematic representation of the interaction between plaque and host tissue.
Natural Factors Favoring Plaque Retention
The formation of a plaque biofilm can be enhanced by natural retention factors, which can also render biofilm removal by means of oral hygiene more difficult. These retention factors include:
• Supra- and subgingival calculus
• Cementoenamel junctions and enamel projections
• Furcation entrances and irregularities
• Tooth fissures and grooves
• Cervical and root surface caries
• Crowding of teeth in the arch.
By itself, calculus is not pathogenic. However, its rough surface presents a retention area for vital, pathogenic bacteria. At the microscopic level, the cementoenamel junction is very irregular, and offers retentive roughness. Enamel projections and “pearls” also inhibit soft tissue attachment. Furcation entrances, fissures, etc. are retentive niches for plaque. Carious lesions represent a huge bacterial reservoir. Crowding of teeth reduces self-cleansing and renders oral hygiene more difficult.
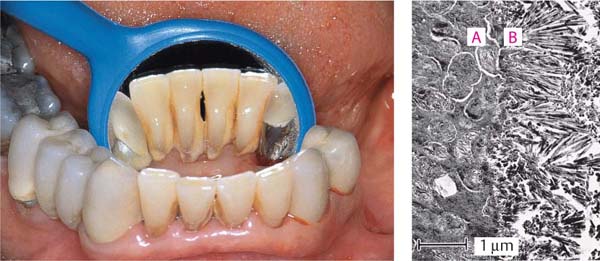
48 Supragingival Calculus
Lingual surfaces of mandibular incisors and buccal surfaces of maxillary molars near the orifices of salivary ducts often exhibit massive accumulations of supragingival calculus.
Right: TEM of old supragingival calculus. Calcified plaque (A) close to the tooth surface. Note the accumulation of cell-free hexagonal monocrystals (B) upon the calcified plaque.
Courtesy H. Schroeder
49 Subgingival Calculus
In this patient with long-standing periodontitis, the gingiva has receded. Calculus that was formerly subgingival is now supragingival.
Right: Subgingival calculus is observed clinically after reflecting the gingival margin. Subgingival calculus is usually dark in color (Fe minerals) and harder than the more loosely structured supragingival calculus (calcium phosphates). The cementoenamel junction is indicated by the dashed line.
50 Crowding, Enamel Projection (enamel “pearl”)
The lingually displaced mandibular incisors do not benefit from the natural self-cleansing action of the lower lip. Oral hygiene is also rendered more difficult.
Right: The furcation on this molar is filled by a projection of enamel that ends in a bulbous pearl, extending into the interradicular area. When a pocket forms in such an area, plaque control is particularly difficult.
Iatrogenic Factors Favoring Plaque Retention
Restorative dentistry—from a simple restoration to a full-mouth reconstruction—can do more harm than good to the patient’s oral health if performed improperly! Placing only optimum restorations is synonymous with preventive periodontics (tertiary prevention, p. 198).
Fillings and crowns that appear to be perfect clinically and macroscopically almost always exhibit deficiencies at the margins when viewed microscopically. When margins are located subgingivally, they always present an irritation for the marginal periodontal tissues.
Overhanging margins of restorations and crowns accumulate additional plaque. Gingivitis ensues. The composition of the plaque changes. The number of gram-negative anaerobes (e. g., Porphyromonas gingivalis), the organisms responsible for initiation and progression of periodontitis, increases rapidly (Lang et al. 1983).
Gross iatrogenic irritants such as poorly designed clasps and prosthesis saddles may exert a direct traumatic influence upon periodontal tissues.
51 Amalgam Restoration—Clinical View and SEM
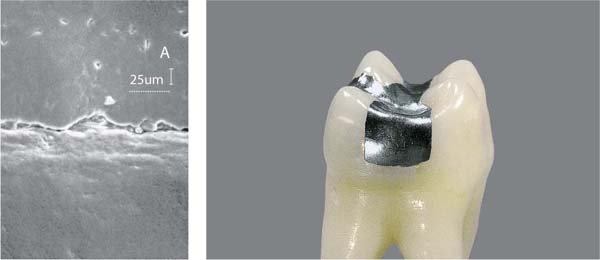
Left: Viewed in the scanning electron microscope, a clearly visible margin defect is observed. Such a defect is a perfect niche for the accumulation of plaque. The amalgam restoration (A) is at the top of this figure, the adjacent enamel below. The white dot under the 25 μm legend are representative of the size of coccoid microorganisms (ca. 1 μm).
Courtesy F. Lutz
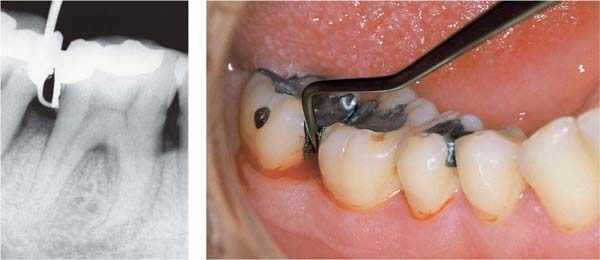
52 Amalgam—Proximal Overhang
Gross overhangs such as this, located subgingivally, invariably lead to plaque accumulation and to gingivitis (note hemorrhage). Pathogenic, gram-negative anaerobes are frequently observed. In contrast to amalgam and especially gold restorations, composite resin restorations are particularly retentive of bacteria.
Left: Radiograph of the same case.
53 Crown Margin Overhang and Open Margins
The cement that was used to cement this crown has begun to extrude from the open margin. The massive retention of plaque between the crown and the prepared tooth leads to severe gingivitis and establishment of a pathogenic bacterial flora.
Left: Section through a porcelain-fused-to-metal crown with a margin that is both overhanging (arrows) and open. Calculus (C) and plaque have accumulated apically.
Subgingival Plaque
Extending apically from the supragingival region, a subgingival plaque biofilm will often form within the existing gingival sulcus/pocket; this was previously called the “adherent” plaque. In addition to gram-positive bacteria such as streptococci, actinomyces, etc., as the probing depth increases so does the number of anaerobic gram-negative bacteria (p. 36).
This subgingival biofilm can also calcify. A dark, hard and difficult to remove calculus (“serum calculus”) accumulates. In addition, the gingival pocket also contains loose agglomerates of non-adherent, often mobile bacteria (with a high concentration of gram-negative anaerobes and spirochetes). In acute phases, periodontopathic bacteria often increase dramatically. These include Actinobacillus actinomycetemcomitans, P. gingivalis, T. forsythensis, spirochetes etc. (pp. 30, 33, 38). Despite these alterations in the subgingival plaque, periodontitis, even in the acute stage, cannot be characterized as a “highly specific” infection because large differences have been reported in the bacterial composition between patients and even within different pocket locations in the same patient (Dzink et al. 1988, Slots & Taubmann 1992, Lindhe 1997).
54 Subgingival Pocket Flora
This is a relatively thin adherent biofilm (blue-violet). One observes loose accumulations of gram-negative, anaerobic, and also motile bacteria. Formations resembling test tube brushes, consisting of filamentous bacteria are also observed (inset).
Histology courtesy M. Listgarten
Right: As pocket depth increases (arrow), the resident flora becomes increasingly gram-negative and anaerobic.
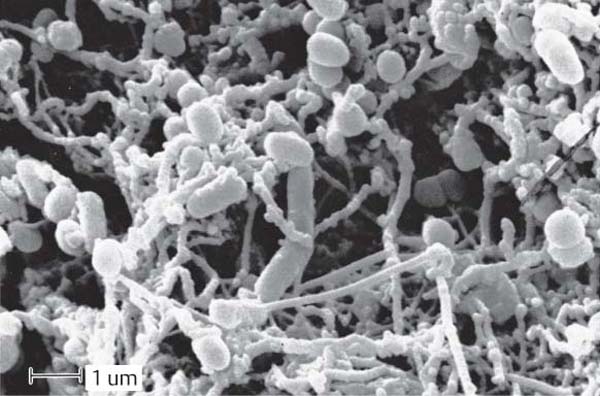
55 Surface of the Biofilm on the Root
Within a pocket, the root surface of a tooth manifesting periodontitis is covered with a densely intertwined bacterial colonization composed of many different bacterial morphotypes (scanning electron photomicrograph).
The morphology of the bacteria permits neither a determination of the species nor any clues concerning pathogenicity.
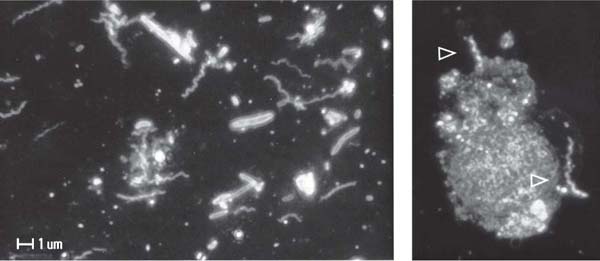
56 Microorganisms of the Non-Adherent Plaque—“Planktonic” Phase
In a dark-field preparation, motile rods and small to larger/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses