“Not for Wrinkles—Botulinum toxin type A has been promoted for use as a wrinkle remedy. Apparently, some practitioners have been injecting the substance to ease wrinkles by weakening face muscles. In a November 18, 1994, Federal Register notice, the FDA denounced the promotion of such unapproved use as ‘an egregious example of promoting a potentially toxic biologic for cosmetic purposes.’ ”
BOTOX
After initial skepticism, the use of botulinum toxin has become the most popular of all surgical or non-surgical cosmetic procedures since its approval in 2002. Currently, botulinum toxin type A is marketed for the reduction of wrinkles as Botox Cosmetic (Allergan Pharmaceuticals, Irvine, Calif). It is FDA approved for reduction of moderate to severe glabellar lines in patients 18 to 65 years of age. Myobloc (Solstice Neurosciences, Malvern, Pa) is a form of botulinum toxin type B that is available in the United States. In Europe, it is available as NeuroBloc. Myobloc is FDA approved for the treatment of patients with cervical dystonia. It has been used (but is not FDA approved) for cosmetic use. Dysport (Ipsen Limited, Slough, United Kingdom) is another brand of botulinum toxin type A that is available outside the United States.
HISTORY AND PHARMACOLOGY
Botulinum toxin is an exotoxin produced by the obligate anaerobe Clostridium botulinum . C. botulinum is a spore-forming, gram-positive rod found in the soil. The exotoxin is an A-B neurotoxin, or type III toxin, that consists of two polypeptides. Part A causes the action of the toxin, and part B binds to the host cell. Of all natural toxins, botulinum toxin is the most potent. Multiple serotypes of the toxin—A, B, C1, C2, D, E, F, and G—are produced by bacteria. Of these serotypes, A is the most potent. Types A, B, and E are the most likely causes of human disease. The toxin binds to the presynaptic neuron of the neuromuscular junction, enters the cell, and acts by inhibiting the release of acetylcholine. This results in neuromuscular blockade and flaccid paralysis.
Note: Type I toxins are superantigens that cause an intense immune response. Type II antigens are membrane-disrupting toxins that disrupt plasma membranes of host cells, causing lysis.
This toxin causes the disease botulism, a form of food poisoning. Botulism has been known for centuries. The name comes from the Latin word “botulus,” which means “sausage.” The disease was noted to occur in people who had eaten sausage. Symptoms of the disease usually occur within a day or two. Nausea is followed by blurred vision, muscle weakness, and, finally, respiratory or cardiac failure. The disease is rare today because nitrites added to food prevent the growth of bacteria. Heating will destroy the toxin, but not the spores. The toxin will not form in highly acidic foods. In adults, competing bacteria prevent formation of the toxin by ingested bacteria. In infants, competing microorganisms have not been established. Many cases of infant botulism have been reported after honey ingestion, so it is recommended that children younger than 1 year of age not be fed honey.
Botulinum toxin was first investigated for therapeutic purposes in the 1970s. Use of Botox for reduction of facial lines was reported in 1992. It is currently used for many conditions in which relaxation of muscles will provide the desired result. Strabismus, blepharospasm, hemifacial spasm, dystonia, hyperhidrosis, headache, and facial wrinkles are several conditions for which botulinum toxin is used currently.
PREPARATIONS
Botox or Botox Cosmetic (botulinum toxin type A) is supplied in 100 unit vials. One unit is the median lethal dose (LD50) in mice. The estimated LD50 for a 70 kg human is 2,800 units. The calculation of units is specific to each brand and cannot be compared with another product. The vial contains vacuum-dried toxin, human albumin, and sodium chloride. Botox is reconstituted with sterile saline. Non-preserved saline is recommended, although some authors have used preserved saline, and others have recommended addition of 1% lidocaine. Various amounts of diluent may be used; resultant doses are shown in Table 31-1 .
| Diluent Added | Resulting Dose |
|---|---|
| 1.0 mL | 10.0 units |
| 2.0 mL | 5.0 units |
| 2.5 mL | 4.0 units |
| 4.0 mL | 2.5 units |
| 8.0 mL | 1.25 units |
The package insert recommends that unopened vials of Botox should be stored at 2° C to 8° C for up to 24 months, and that reconstituted Botox should be stored under the same conditions and used within 4 hours. However, Garcia and Fulton noted that aged reconstituted Botox worked almost as well as freshly reconstituted Botox. A typical dose of Botox for the treatment of glabellar furrows is 20 units. Results are seen within 1 to 2 days, with increasing intensity noted in the first week.
Myobloc (botulinum toxin type B) is supplied as a sterile liquid. Each vial contains toxin, human albumin, sodium succinate, and sodium chloride. Three different sizes are available: 2,500 units (0.5 mL), 5,000 units (1.0 mL), and 10,000 units (2.0 mL). The dose range for the treatment of glabellar furrows is 1,800 to 3,500 units. Myobloc is not FDA approved for cosmetic use, but several studies have shown its effectiveness. Myobloc (pH 5.6) is more acidic than Botox (pH 7.2), and its duration of action is shorter. Botulinum toxin type B can be effective in patients who develop a tolerance to type A toxin, and it may have a faster onset of action.
Dysport (botulinum toxin type A) is supplied in 500 unit vials as a lyophilized powder that must be reconstituted. Other constituents include human albumin and lactose. The dose range for the treatment of glabellar furrows is 40 to 60 units. This agent is not available in the United States.
USES IN FACIAL COSMETICS
As was mentioned previously, Botox is the only botulinum toxin preparation that has been approved for cosmetic use in the United States, and it is approved only for use in the glabellar area. Any other use is considered off-label. More and more uses are being identified and have been described in peer-reviewed journals.
Glabella
Treatment of glabellar furrows focuses on the corrugator supercilii and procerus muscles. Contraction of the corrugators and to a lesser degree the medial orbicularis oculi and the depressor supercilii produces vertical lines between the eyebrows. Contraction of the procerus produces horizontal lines over the bridge of the nose. The package insert for Botox Cosmetic recommends five injections of 0.1 mL (20 units total), with two injections into each corrugator and one into the procerus. Higher or lower doses can be used in keeping with the muscle strength of individual patients. In general, higher doses are needed for male patients. It is recommended that injections not be made closer than 1 cm to the orbital rim because of concerns about diffusion ( Figure 31-1 ).
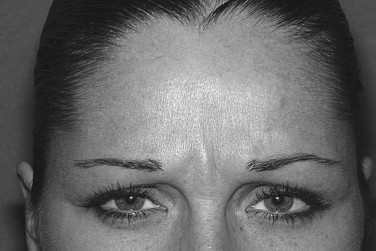
Forehead
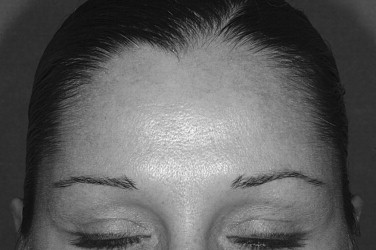
The frontalis muscle produces horizontal forehead lines. The frontalis muscle has two bellies that are separated more superiorly than inferiorly. Injecting into the central tendon will have some effect because of diffusion but will not be as effective as injecting into the muscle itself. Evaluation of the patient to determine whether the frontalis is being used to elevate a ptotic brow is necessary prior to treatment. Unless a combination of procedures is planned, reduction of forehead lines with the result of brow ptosis should be avoided. The amount of Botox used will vary widely depending on the desired results. Relaxation of the entire frontalis muscle usually is not the goal. The lateral frontalis muscle is avoided to prevent brow ptosis. In the more medial area, slight relaxation is usually enough to decrease lines without completely paralyzing the muscle ( Figure 31-2 ).
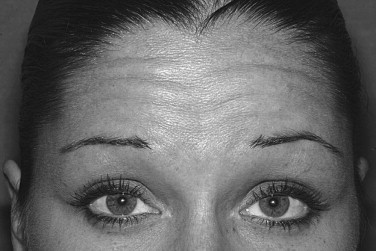
Crow’s Feet
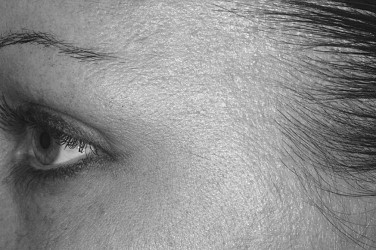
Lateral canthal lines are caused primarily by contraction of the lateral orbicularis oculi muscles. Contraction of the zygomaticus major can cause lines in the lower lid/upper cheek. As with the glabella, injections should be made 1 cm away from the orbital rim. If the zygomaticus major is to be injected, the patient should be warned that this can affect the smile line. Typically, a dose of 6 to 12 units is used on each side ( Figure 31-3 ).
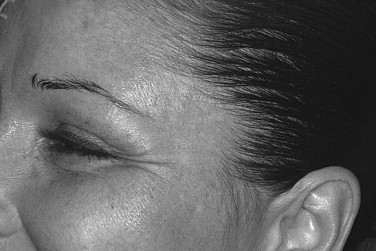
Nasolabial Folds
Relaxation of the levator labii superioris alaeque nasi can decrease the depth of the medial nasolabial fold and the height of the central smile line. This must be done with caution, and the patient must be warned of changes to the smile. The dose used is 2.5 to 7.5 units per side.
Upper Lip
Perioral rhytides are caused by contraction of the orbicularis oris. These wrinkles often are addressed through resurfacing procedures or filler materials. These procedures can reduce the appearance of the rhytides but do not address the underlying cause. Doses of 2 to 6 units given at several injection points or use of the threading technique will slightly relax the muscle. Care must be taken because higher doses can cause lip incompetence.
Chin
Dimpling of the chin can be improved by injection of the mentalis. This technique also has been used to correct abnormal contraction following chin implant or genioplasty ( Figure 31-4 ). With mentalis dysfunction following surgery, deeper placement with a higher dose may be required. Doses range from 2.5 to 12.5 units for dimpling of the chin. Care should be taken to avoid the lip depressor muscles because, as with the upper lip, lip incompetence can result.
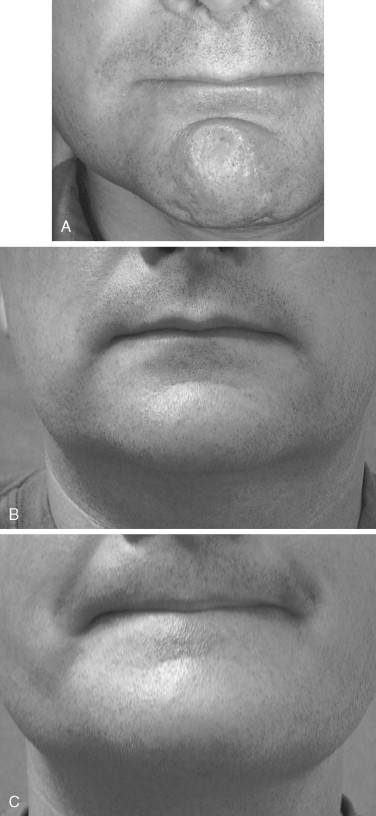
Neck
Hypertrophic platysma muscle banding is a frequent complaint among aging female patients. Patients with this condition can be treated surgically, but good results can be obtained with the use of Botox in patients without significant skin excess. Doses of 5 to 20 units per band injected directly into the muscle band usually will reduce both active and relaxed platysmal bands.
TREATMENT TECHNIQUE
The patient should be evaluated and risks and expected outcomes explained. A consent form should be signed and preoperative photographs taken. The skin should be prepared with an alcohol swab. Application of topical anesthetic or ice may be desired by some patients. Having the patient flex the muscles planned for treatment will guide the injection. For the glabella, the patient should be asked to frown; for the forehead, the patient should be asked to raise the eyebrows; and for crow’s feet, the patient should be asked to smile or squint. Botox should be prepared to the desired dilution and drawn into a syringe. Use of a ½ mL insulin syringe with a marking at each 0.01 mL allows for easy measurement of units of Botox based on dose (see Table 31-1 ). Botox should be injected directly into the muscle. Activating the muscle has been shown to increase uptake of Botox. Botox will diffuse to other areas to some degree. This effect is increased by massage and the use of higher volumes. Some authors recommend massaging areas such as the forehead to disperse the toxin locally. This should be avoided in areas where dispersion will lead to undesirable results, such as near the orbits. Following the procedure, some recommend no activity for several hours. Others recommend that the patient should remain upright. Effects are usually seen within 24 hours, and the intensity will increase to full effect within a week ( Figures 31-5 and 31-6 ). Effects of the medication last for about 3 to 4 months, but with regular treatment, the effects often can last much longer. This may be due to atrophy of the treated muscles or to changes in habits.
CONTRAINDICATIONS AND ADVERSE EFFECTS
As with all cosmetic procedures, patients who have unrealistic expectations should not receive Botox. Patients who may be allergic to any of the components should not receive Botox. Botox is a category C drug that is not recommended during pregnancy. It is not known whether Botox is secreted in breast milk. Patients with neuromuscular disorders (amyotrophic lateral sclerosis, myasthenia gravis, Eaton-Lambert syndrome) may be extremely sensitive to systemic effects, especially dysphagia. Drugs that interfere with neuromuscular transmission (curare, lincosamides, polymyxins, quinadine, magnesium sulfate, anticholinesterases, succinylcholine) may potentiate the effects of Botox.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


