Fig. 3.1
The process of new bone deposition does not cause displacement by pushing against the articular contact surface of another bone. Rather, the bone is carried away by the expansive force of all the growing soft tissues surrounding it. As this takes place, new bone is added immediately onto the contact surface, and the two separate bones thereby remain in constant articular junction. The nasomaxillary complex, for example, is in contact with floor of the cranium (top). The whole maxillary region is displaced downward and forward away from the cranium by the expansive growth of the soft tissues in the midfacial region (center). This then triggers new bone growth at the various sutural contact surfaces between the nasomaxillary composite and the cranial floor (bottom). Displacement thus proceeds downward and forward as growth by bone deposition simultaneously takes place in an opposite upward and backward direction (i.e., toward its contact with the cranial floor) (From Enlow (1975))
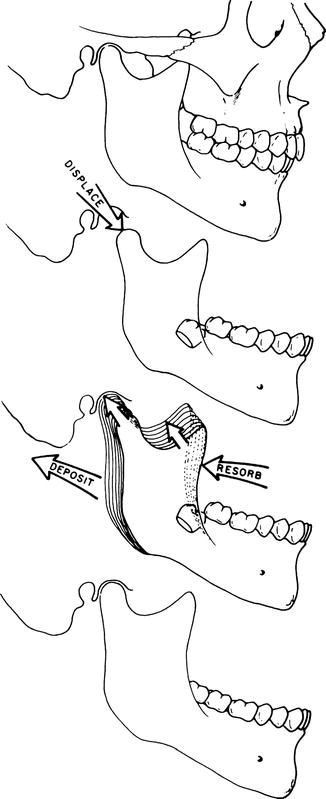
Fig. 3.2
Similarly, the whole mandible is displaced “away” from its articulation in each glenoid fossa by the growth enlargement of the composite of soft tissues in the growing face. As this occurs, the condyle and ramus grow upward and backward into the “space” created by the displacement process. Note that the ramus “remodels” as it relocates posteriorly. It also becomes longer and wider to accommodate (1) the increasing mass of masticatory muscles inserted onto it, (2) the enlarged breadth of the pharyngeal space, and (3) the vertical lengthening of the nasomaxillary part of the growing face (Reprinted with permission from Enlow (1975))
Enlow (1975) goes on to explain that, with the development of the functional matrix principle, a number of important hypotheses began to receive attention. One of these is that the “bone” does not regulate its own growth. The genetic and epigenetic determinants of skeletal developments are in the functional tissue matrix, that is, muscle, nerve, glands, teeth, neurocranial fossa, and nasal, orbital, oral, and pharyngeal cavities. This is primary while the growth of the skeletal unit is secondary. However, although the functional matrix principle describes what happens during growth, it does not account for how it happens. Experiments have shown that mechanical forces are not the principal factor controlling bone growth.
Most researchers agree that a notable advance was made with the development of the functional matrix principle introduced by Moss (1962, 1969). It deals with what determines bone and cartilage growth in general. The concept states, in brief, that any given bone grows in response to functional relationships established by the sum of all the soft tissues operating in association with that bone. This means that the bone itself does not regulate the rate and direction of its own growth; the functional soft tissue matrix is the actual governing determinant of the skeletal growth process.
The course and extent of bone growth are secondarily dependent on the growth of pace-making soft tissues. Of course, the bone and any cartilage present are also involved in the operation of the functional matrix, because they give essential feedback information to the soft tissues. This causes the soft tissues to inhibit or accelerate the rate and amount of subsequent bone growth, depending on the status of the functional and mechanical equilibrium between the bone and its soft tissue matrix. The genetic determinants of the growth process reside wholly in the soft tissues and not in the hard part of the bone itself.
The functional matrix concept is fundamental to an understanding of the overall process of bone growth control. This concept has had a great impact in the field of facial biology. The concept also comes into play as a source for the mechanical force that carries out the process of displacement. According to this now widely accepted explanation, the facial bones grow in a subordinate relationship with all the surrounding soft tissues. As the tissues continue to grow, the bones are passively (i.e., not of their own doing) carried along (displaced) with the soft tissues attached to the bones by Sharpey’s fibers. Thus, for the nasomaxillary complex, the expansion of the facial muscle, the subcutaneous and submucosal connective tissues, the oral and nasal epithelia lining the spaces, the vessels, and the nerves all combine to move the facial bones passively along with them as they grow. This continuously places each bone and all of its parts in correct anatomic positions to carry out its functions. Indeed, the functional factors are the very agents that cause the bone to develop into its definite shape and size and to occupy the location it does.
Growth control is determined by genetic influences and biomechanical forces, but the nature of the balance between them is still, at best, uncertain. No single agent is directly responsible for the master control of growth; the control process encompasses many factors. It involves a chain of regulatory links. Moreover, not all of the individual links are involved in all types of growth changes.
Enlow (1975) identifies the maxillary tuberosity as being a major site of maxillary growth. It does not, however, provide for the growth of the whole maxilla, but rather is responsible for the lengthening of the maxillary arches. The whole maxilla is displaced in an anterior direction as it grows and lengthens posteriorly. However, the nature of the force that produces this forward movement is a subject of great controversy. The idea that additions of new bone on the posterior surface of the elongating maxillary tuberosity “push” the maxilla against the adjacent pterygoid plates has been abandoned.
Bones do not by themselves have the physiological capacity to push away bones. Another theory held that bone growth at the various maxillary sutures produces a pushing apart of the bones, with a resulting thrust of the whole maxilla downward and forward. This theory has also been rejected because bone tissue is not capable of growth in a field that requires the amount of compression needed to produce a pushing type of displacement. The sutural connective tissue is not adapted to a pressure-related growth process. It is believed that the stimulus for sutural bone growth is the tension produced by the displacement of the bone. Thus, the deposition of new bone is a response to displacement rather than the force that causes it. Although the “sutural push theory” is not tenable, Enlow reports that some students of the facial growth control processes are looking anew at growth mechanizing sutures, but not in the old conceptual way.
3.1.4 Cartilage-Directed Growth: Nasal Septum Theory (Scott 1953, 1954, 1955, 1956a, b, 1957, 1958a, b, 1959)
Cartilages are the leading factor. Synchondrosis, nasal septum, and mandibular condyles are actual growth centers. Sutural growth is compensatory. This theory developed from criticisms of the “sutural theory.” Scott (1953, 1954) believes that cartilage is specifically adapted to certain pressure-related growth sites because it is a special tissue uniquely structured to provide the capacity for growth as a result of compression. The basis for this theory is that the pressure-accommodating expansion of the cartilage in the nasal septum is the source of the physical force that displaces the maxilla anteriorly and inferiorly. This, according to Scott’s hypothesis, sets up fields of tension in all the maxillary sutures. The bones then, while they enlarge at their sutures in response to the tension created by the displacement process, move in relation to each other.
The nasal septum hypothesis was soon adopted by many investigators in cleft palate centers around the world and became more or less the standard explanation, replacing the “sutural theory.” Clinicians involved in cleft palate treatment, such as McNeil (1950, 1954, 1964) and Burston (1960) and their followers (Crikelair et al. 1962; Cronin and Penoff 1971; Derichsweiler 1958; Dreyer 1962; Georgiade 1970; Georgiade and Latham 1975a, b, Graf-Pinthus and Bettex 1974; Hellquist 1971; Huddart 1979; Kernahan and Rosenstein 1990; Krischer et al. 1975; Latham 1968; Robertson 1971; Monroe and Rosenstein 1971), accepted Scott’s thesis that cartilage and periosteum carry an intrinsic genetic message that guides their growth. They believed that the cartilaginous centers, such as the chondrocranium, the associated synchondroses, and the nasal septum, should be viewed as the true centers of skull and facial growth. Scott (1953, 1954) further suggests that the nasal septum plays more than a secondary role in the downward and forward vector of facial growth.
McNeil (1950, 1954), following Scott’s thesis, describing the embryopathogenesis of complete clefts of the lip and palate and their treatment at the neonatal period, wrote that the palatal processes, being detached from the growing nasal septum, do not receive their growth impetus and, therefore, are not only retruded within the cranium but are also deficient in osteogenic tissue. He goes still further and believes that the deficient palatal processes can be stimulated to increased size through the use of functional orthopedics.
3.1.4.1 Stimulation of Bone Growth: Is It Possible?
As McNeil saw it, pressure forces created by “functional” orthopedic appliances, which are within the limits of tolerance, will act to stimulate bone growth in an anterior direction. This force needs to be applied to particular regions and in particular directions so that it can intensify normal forces. The resulting narrowing of the cleft is due to growth of the underlying bone brought on by such stimulating appliances. Additional growth leads to a reduction in the soft palate cleft as well, thereby increasing the chance of having a long, flexible, well-functioning soft palate after surgical closure.
McNeil (1954) goes on to suggest that an obturator alone is unsatisfactory because it will reduce “valuable” tongue space and lead to harmful speech habits. McNeil was correct in stressing that surgery should be reduced to a minimum compatible with sound clinical reasoning and accepted surgical principles.
Whereas McNeil states that his procedure stimulates palatal growth, thereby narrowing the cleft space, Berkowitz’s (1989) 3D palatal growth studies – using a sample of cases that have not had neonatal maxillary orthopedic treatment and a control sample of noncleft cases – show that growth occurs spontaneously. This is an expression of the palate’s inherent growth potential, which can vary among patients. Berkowitz concluded that “catch-up growth” can occur after palatal surgery (with minimum scarring) is performed.
3.1.4.2 The Need to Prevent Collapse
McNeil (1950, 1954, 1964) further believes that the palatal segments should be manipulated to an ideal relationship prior to lip surgery to prevent them from moving too far medially and becoming collapsed with the buccal segments in crossbite. This, he suspects, will lead to abnormal movements of the tongue and give rise to faulty respiratory, sucking, and swallowing patterns, also causing abnormal growth and development of the palatal structures.
Mestre et al. (1960), studying palatal size in a cleft population that had not been operated on, report that the development of the maxilla appears to be normal in unoperated cases. They do conclude that it is the type, quality, and extent of the surgery that determine the effect on maxillary growth and that osteogenic deficiency does exist to varying degrees. Our research on serial palatal growth changes supports this conclusion that palates with clefts are highly variable in size, shape, and osteogenic deficiency.
Unfortunately, McNeil’s interpretation of the effects of clefting on the various vegetative functions, and in reducing palatal growth, has not been supported by controlled objective research. The inability of the manipulated arch to remain intact after lip surgery, and not move medially into a collapsed relationship, has led many clinicians to question the accuracy of McNeil’s other stated benefits such as reduction of middle ear infections.
McNeil (1950, 1954, 1964) made other faulty observations. Among them:
1.
He mistakenly believed that the orthopedic appliance will stimulate the underdeveloped cleft segment in unilateral clefts of the lip and palate (UCLP) to move forward, to make contact with the premaxillary portion of the greater segment and both palatal segments in bilateral clefts of the lip and palate (BCLP), after the lip is united. Even as early as the 1960s, many orthodontists found the opposite to be true. In UCLP, the premaxillary portion of the larger segment moves medially and backward to make contact with the lesser segment due to the action of compressive lip muscle forces. If McNeil had had the benefit of serial casts, his interpretation of clinical events would, I am confident, have been totally different.
2.
McNeil’s claim that the lesser segments in UCLP, and both segments in BCLP, can be stimulated to grow forward is totally erroneous. His conclusions were based on conjecture, not on objective data. The results of Berkowitz’s 3D palatal growth studies (Wolfe and Berkowitz 1983) show marked acceleration in palatal growth during the first 2 years without orthopedic treatment, with most of the growth changes occurring at the area of the maxillary tuberosity and not at the anterior portion of the palate except for alveolar growth associated with canine development (Fig. 3.2). Movement of the cleft palatal segment anteriorly is only possible as a result of reactive mechanical forces being applied through the use of pinned maxillary orthopedic appliances or from a protraction facial mask.
One last but significant characterization of a newborn cleft of the lip and palate needs to be refuted. McNeil states that “in BCLP lateral segments are collapsed toward the midline before birth.” However, he does not explain the dynamics that can make this possible. How can segments be collapsed if there are no inwardly directed forces from the cleft lip–cheek muscle complex, especially when the tongue fits within the cleft space and acts to move the palatal segments apart?
Enlow’s (1975) report on current thinking on palatal growth processes delivers McNeil’s thesis a mortal blow. Enlow (1975) writes that recent research has shown that pressure is detrimental to bone growth.
Bone is necessarily both a traction and pressure-adapted kind of tissue. The periosteal membranes are constructed to function in a field of tension (as by the pull of a muscle). Covering membranes are quite sensitive to direct compression because any undue amount causes vascular occlusion and interference with osteoblastic formation of new bone. Osteoclasts, conversely, function to “relieve” the degree of pressure by removing bone. Bone is pressure sensitive, and high-level pressure induces resorption.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


