(1)
Canberra, ACT, Australia
Summary
The periodontal tissues and pulp–dentine complex form an intimate continuum through which pathological changes of either one may lead to infection of the other. The management of such lesions can be fraught with diagnostic and therapeutic difficulty requiring a methodical multidisciplinary approach. Traditional classifications of endodontic–periodontal lesions are largely academic and based inappropriately on an attempt to identify the primary source of infection. Treatment and prognosis of endodontic–periodontal lesions depend on the cause and the correct diagnosis of each tissue. It is critical to determine whether the lesion is primarily endodontic or periodontal in origin since this will determine which treatment plan is instigated. True combined lesions require a staged approach with endodontic treatment initiated followed by a 2–3-month review to reassess outcome. Appropriate periodontal therapy can then be initiated followed by further review to assess outcome. The long-term prognosis for such cases will be guarded requiring not only careful clinical management but also patient motivation.
Clinical Relevance
A peri-apical lesion of pulpal origin may simulate the radiographic appearance of advanced periodontal disease and drain through the sinus tract originating from the apex, lateral or furcal accessory canal along the root surface. This condition is not a true periodontal pocket and must be distinguished from those of periodontal origin. Accurate diagnosis is usually attained through careful diagnostic testing including pulp vitality, periodontal probing and radiographic examination. In addition to lesions of endodontic origin, palatal grooves, fused roots, enamel pearls and vertical root fractures can cause narrow probing defects as well. The long-term success of a true combined endodontic–periodontal lesion depends on a number of crucial and key factors including the severity and extent of the initial peri-apical and periodontal infections, the correct treatment planning and decision-making, the skill and experience of the clinician(s) and the motivation of the patient, particularly with long-term periodontal care
16.1 Overview of Endodontic–Periodontal Interrelationship
Endodontic–periodontal lesions refer to diseases, which affect the peri-apical and periodontal tissues through microbial infections arising from the root canal system, periodontal pocket or a combination of both. Such infections are a challenge to the clinician as far as diagnosis, prognosis and decision-making are concerned. In particular, it is critically important to determine whether the lesion is primarily periodontal or primarily endodontic in origin, because the accuracy of diagnosis will determine whether or not the appropriate treatment plan is instigated [1].
A number of classification systems have been proposed resulting in confusion and controversy due to overlapping terminology. The majority have focused on identifying the initial source of infection, which can be difficult. Classification systems are necessary as they guide the diagnoses, which, in turn, determine prognosis and the most appropriate sequence of treatment. Furthermore, classification systems allow similar clinical conditions to be evaluated in clinical trials so that the evidence can be translated into clinical practice. Discussing prognosis with patients prior to treatment allows patients to understand all the risks involved with a proposed treatment and gives the opportunity to decide whether a tooth is treated or removed [2, 3].
An excellent systematic review was recently published highlighting the variability of definitions for PE lesions as well as the small number of controlled clinical trials [4]. PE lesions were defined as the coexistence of a negative or altered pulp vitality test and a probing pocket depth (PPD) ≥6 mm (on at least one tooth for case reports, or mean for interventional studies and case series). The majority of publications are case series that demonstrate successful treatment outcomes following an accurate diagnosis and staged treatment. These sequential treatment protocols have become the mainstay of treatment for PE lesions. Future studies should be well-designed, randomised, controlled, clinical trials to reduce the publication bias inherent within the case series with negative results less likely to be published [5]. Within the studies analysed in the systematic review, there was a high number of patient dropouts (up to 27.9 %) with the outcome of treatment not truly evaluated. Therefore, care must be taken in treatment planning PE lesions, as prognosis may be difficult to determine from the outset and during treatment. Extended periods of observation may be useful in assessing the outcome of periodontal treatment prior to a final decision on completion of endodontic care or placement of a full-coverage restoration [42].
In both endodontic and periodontal diseases, the primary aetiology for causing disease is microbial. In a classical study, the pulps of normal rats were exposed and left open to the oral environment, resulting in pulpal necrosis, peri-apical inflammation and peri-apical lesion formation. However, when the same procedure was performed in germ-free rats, pulps not only remain vital with minimal inflammation but also dentinal repair at the exposure site occurred. The study demonstrated that without bacteria and their by-products, peri-apical lesions of endodontic origin did not occur [6]. In pulpal disease, the pathogens are located as biofilm within the internal walls of the root canal system, whereas in periodontal disease, the biofilm is located on the external root surface. Using specific PCR methods, the profiles of periodontal pathogens in pulpal and periodontal disease within the same tooth have been used to confirm the predominant anaerobic species present [7]. Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia and Treponema denticola were found in all endodontic samples, and the same pathogens were found in teeth with chronic apical periodontitis and chronic adult periodontitis [7].
Several main avenues for exchange of infectious elements and irritants (microbial) between the pulp space and periodontium exist including the apical foramen, lateral and accessory root canals, furcation area and furcal canals, apical delta and dentinal tubules. The apical foramen represents the largest communication between the pulp and the peri-apical tissues. It allows the passage of endodontic bacteria and by-products into the periodontal tissues to form apical lesions [6]. Similarly, periodontitis advancing towards the apex can disturb the apical blood supply and lead to pulpal necrosis. In some situations, bone loss from periodontitis can extend beyond the root apex mimicking the radiographic appearance of an apical radiolucency caused by an infected pulp. This highlights the importance of combining radiographic findings with clinical examination and sensibility tests. If there is no obvious cause for the tooth becoming nonvital (such as caries), the restoration should be removed to allow proper assessment to rule out fractures or cracks. The use of an endodontic microscope may aid diagnosis and also allows teeth with unrestorable fractures to be removed prior to embarking on treatment [8].
Lateral canals occur due to the breakdown of Hertwig’s epithelial root sheath during root formation [9]. Blood vessels running between the dental papilla and dental follicle may be retained and form accessory canals. Around 27 % of teeth have lateral canals that are more commonly found in the apical areas of the root [10]. A study of 100 extracted human teeth found that only 2 % of lateral canals were associated with periodontal pockets when advanced periodontitis was present [11]. This histological study supports the clinical findings that only 3–4 % of teeth with advanced periodontitis require endodontic treatment [12, 13]. If a lateral canal is exposed to the oral environment, a chronic inflammatory lesion may develop. Whilst pulp necrosis is possible [14], for the majority of cases, the apical blood supply remains intact and explains why pulp necrosis is not common. Studies have also shown that lateral and accessory canals do not always transverse the entire length of the dentine and that many are filled with connective tissue attachment rather than blood vessels [15].
When dentine is exposed to the oral environment, through caries or a periodontal pocket, the tubules can be penetrated by bacteria [16]. When the pulp is healthy, the outflow of dentinal fluid may limit bacterial penetration to the outer 300 μm of the dentine [17]. Furthermore, prolonged bacterial insult leads to a protective pulpal reaction, formation of secondary dentine and narrowing or occlusion of the dentine canal at the pulp wall. The narrowing of tubules combined with the outflow of dentinal fluid prevents bacterial ingress into the pulp [17]. Therefore, it is unlikely that periodontitis will lead to endodontic disease unless larger communications are present. Root planing can mechanically dislodge bacteria into the tubules and may explain the presence of bacteria in dentinal tubules.
Besides these normal anatomical avenues, the pulp and periodontal tissues may communicate through either direct pathological pathways such as vertical root fracture [18] and root perforation [19] or indirectly via developmental anomalies such as deep radicular grooves [20].
Periodontitis may have a cumulative effect on the pulp tissue once lateral canals are exposed. These degenerative changes may manifest as calcifications, resorption or inflammation leading to damaged pulp tissue [21]. Langeland and co-workers demonstrated that total disintegration might occur when all the main apical foramina are involved [22]. In a primate study, the investigators found that scaling and root planing, and subsequent plaque re-accumulation on the exposed root dentin, does not cause severe alteration in the pulp [23]. This was confirmed in additional studies that demonstrated that the incidence of endodontic disease in cases of advanced periodontitis was small [24–26]. Some of the best evidence arises from studies evaluating pulpal histology of roots that were resected due to very advanced periodontitis and bone loss near the apex. Smukler and Tagger found that none of the 20 samples showed inflammatory changes [26]. Similarly, Haskell found very few inflammatory cells in the pulps of resected roots. This suggests that periodontitis does not have a significant effect on the pulp even when bone loss is extensive [25].
An abscess is a localised collection of pus in a cavity formed by disintegration of tissues. The formation of pus is termed suppuration. The endodontic abscess occurs after necrosis of the dental pulp and subsequent root canal infection. The periodontal abscess occurs after infection of the periodontal tissues by bacteria of the subgingival microbiota. Dental abscesses in general expand through tissues providing the least resistance by forming a sinus tract (fistula). In the case of a periodontal abscess, drainage is most likely to take place through the periodontal pocket. In the case of a peri-apical abscess, the spread is primarily dictated by the thickness of overlying cortical bone and the location of the abscess in relation to the muscle attachments. In periodontal health, the fistula may occasionally drain from a peri-apical abscess along the root surface into the gingival pocket. It is no different from a draining sinus tract in the alveolar mucosa or attached gingivae. When the pocket is probed, it is narrow and lacks width. From a diagnostic point-of-view presentation, pulp testing procedures and periodontal probing are critical to accurate diagnosis (see Table 16.1). Insertion of a gutta-percha cone into the sinus tract and taking subsequent radiographs can help determine the origin of the lesion. No periodontal treatment is indicated and it should resolve following adequate endodontic therapy [14, 27, 28].
Table 16.1
Differential diagnosis of a lateral periodontal and apical abscess
|
Lateral periodontal abscess
|
Apical abscess
|
|
|---|---|---|
|
Pain
|
Less severe than apical abscess
|
Severe
|
|
Swelling
|
More gingivally
|
Usually over apex
|
|
Palpation tenderness
|
More gingivally
|
Apically
|
|
Percussion tenderness
|
Mild or none
|
Very tender
|
|
Timing
|
Usually swelling before pain
|
Usually pain before swelling
|
|
Pocket formation
|
Yes
|
Not always but can occur
|
|
History of trauma/previous restoration
|
Not usually
|
Usually
|
|
Previous symptoms of pulpitis
|
Not usually
|
Frequently
|
|
Radiographic appearance
|
Marginal bone loss evident
|
May be apical rarefaction
|
When an endodontic infection drains through a pre-existing periodontal pocket (periodontal disease), the pre-existing periodontal pocket appears more extensive because of the draining endodontic lesion. Whilst endodontic treatment results in periodontal healing at the base of the pocket, periodontal treatment will be required to treat any residual pocketing.
Animal studies have demonstrated that periodontal debridement of root surfaces exposed due to an endodontic infection results in down-growth of epithelium and compromised healing [29]. This relates to the removal of periodontal ligament and cementum on the root surface during scaling and root planing, and therefore, the potential for reattachment is lost [30]. In more severe cases where endodontic infection meets a pre-existing advanced periodontal pocket, treatment can be carried out in short succession. For example, root resection may be performed if it is felt that the periodontal infection would cause recontamination of the apical area. However, in most cases endodontic treatment should be applied first.
When endodontic therapy has been completed to an acceptable standard, there does not appear to be any difference in the stability of marginal bone levels [31] with more recent studies showing no impact of the success of periodontal regeneration in root-filled teeth [32]. The series of studies by Jansson and co-workers evaluated the impact of endodontic status in patients treated for advanced periodontitis. Deeper periodontal pocket depths were found in teeth with peri-apical lesions, defined by loss of lamina dura or a widened periodontal ligament space in teeth with or without previous endodontic treatment. The clinical relevance is unclear as the difference in probing depths was less than 1 mm. When examined longitudinally, the amount of bone loss was higher in sites adjacent to apical lesions (0.19 mm/year. vs. 0.06 mm/year). These studies confirm the need to treat endodontic infections prior to periodontal treatment; however, the clinician needs to be cautious not to treat a radiolucency that is not associated with endodontic infection. Review of previous radiographs and consideration of clinical findings and diagnostic tests will help reduce the risk of overtreatment [33–35].
Endodontic–periodontal lesions are classified according to the origin of the problem. There are various definitions in the literature causing a great deal of confusion [1–3, 14, 28]. Accurate diagnosis is essential and will establish the nature and timing of treatment. The most important feature of classifying periodontal–endodontic lesions is whether there is communication, which forms the basis of a true combined lesion (see Figs. 16.1 and 16.2).
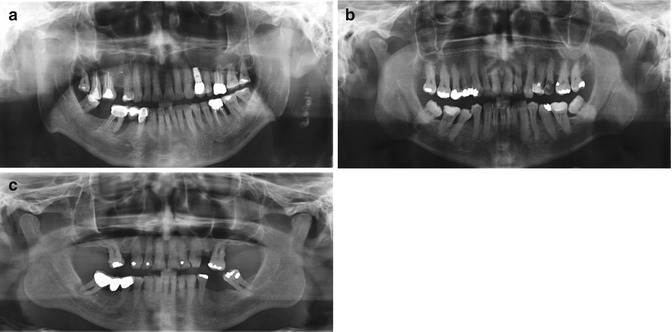
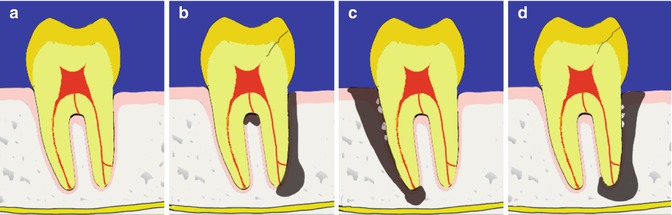

Fig. 16.1
(a–c) Clinical radiographs demonstrating moderate to severe periodontitis with concurrent endodontic pathology

Fig. 16.2
Diagrams representing classification of periodontal–endodontic lesions (a) Normal healthy tooth. (b) Primary endodontic lesion (either peri-apical, lateral or furcal origin). (c) Primary periodontal lesion. (d) True combined lesion. Different treatment approaches will be dictated by origin of lesion
Primary periodontal lesions represent an advanced periodontal lesion that has disrupted the blood supply to the apex of the tooth causing it to become nonvital. This is rare with studies reporting this to occur in 3–4 % of teeth with advanced periodontitis [12, 24]. These lesions have a poor prognosis due to the difficulty in debriding the root surface near the apex and the extent of periodontal destruction.
Primary endodontic lesions occur when an endodontic infection causes periodontal destruction. This may be caused by drainage of an endodontic infection through a periodontal pocket. Overall, these lesions have a good prognosis if the endodontic cause can be addressed. A primary endodontic lesion can also arise due to communication between the pulp and periodontium following endodontic perforations, resorptions and root fractures. The prognoses of these types of lesions can be questionable.
True combined periodontal–endodontic lesions form when there is coalescence of endodontic and periodontal lesions [36]. It is not possible to accurately determine the prognosis of the tooth at the initial examination because the relative contribution from endodontic versus periodontal infection is unknown. Once the endodontic infection has been eliminated, the residual periodontal defect that remains can guide overall tooth prognosis. Therefore, if a true combined lesion is suspected, an endodontic dressing is placed after irrigation and debridement of the canals. Following endodontic healing, the periodontal pockets are reassessed to determine feasibility of periodontal treatment in order to retain the tooth. True combined periodontal–endodontic lesions will require endodontic therapy, periodontal treatment (possibly periodontal surgery) and a full-coverage restoration, requiring a significant investment in treatment time and costs. This cost must be weighed up against the patient’s wishes to keep the tooth at all costs versus the prognosis of any tooth replacement. An additional consideration is the difficulty to assign an accurate periodontal prognosis [37], and therefore, there may be a tendency to extract periodontally involved teeth because of this uncertainty. However, we must acknowledge the potential for biological complications [38] with patients with a history of periodontitis having a higher susceptibility for peri-implantitis [39]. Bragger and co-workers also showed that one in three patients experienced a technical implant complication within the first 10 years of treatment [40]. Patients need to be involved in the treatment planning process so that the risk of failure is minimised.
Longitudinal tooth cracks and fractures that principally occur in the vertical plane or long axis of the crown and/or root can lead to particular uncertainty in terms of diagnostic and treatment decisions. The term ‘crack’ implies an incomplete break in the tooth exists, whereas the term ‘fracture’ implies an incomplete or complete break in the tooth exists. When either a crack or fracture has been present for a particular time period along the long axis of the tooth, it can be classified as a longitudinal fracture [48].
Longitudinal fractures can be occurring in both anterior and posterior teeth as a result of multifactorial aetiology. Predisposing factors such as loss of healthy tooth substance due to caries or trauma can increase the risks of cracks that can propagate to fracture. Physical trauma, occlusal prematurities, repetitive heavy and stressful chewing have also been identified as risk factors [49–51]. Complex restorative procedures including endodontic access preparation, loss of marginal ridges, intra-canal dowel placement and excessive dentine removal can all result in weakened tooth structure increasing susceptibility to cracks or fractures [52]. Anatomical predisposition such as narrow mesio-distal dimensions of premolar teeth and the mesial roots of mandibular molars make these teeth more susceptible to fracture, particularly if additional tooth structure is removed during root canal or post preparation procedures [53–56].
Identification of longitudinal root fractures can be difficult. Cracks or fractures that have been present for extended period of times may become stained, cause pain or result in obvious bone loss that can be diagnosed both clinically and radiographically. Techniques available for detection of fractures include transillumination [55], biting devices such as the Fracfinder, methylene blue staining, magnification and illumination and radiography [57]. Clinical signs and symptoms as well as radiographic features can often be similar to those associated with non-healing endodontic treatments. Certain periodontal manifestations including accompanying bone loss, pocketing and sinus tracts may be present. Unless the crack or fracture is obviously visible by direct inspection, then the diagnostic dilemma of whether these features are associated with endodontic pathology, periodontal pathology, a true combined lesion or longitudinal fracture remains. Nevertheless a rapid decision is required to avoid further bone loss, which can lead to reconstructive difficulties particularly if a dental implant is planned in the future [58].
The American Association of Endodontists has categorised longitudinal root fractures into five major classes: craze lines, fractured cusp, cracked tooth, vertical root fracture and split tooth. Craze lines affect only the enamel, originate on the occlusal surface arising from occlusal forces or thermocycling and are asymptomatic. Fractured cusp occurs on the cusps and cervical margins of the root resulting in acute pain on mastication and thermal stimulus. Cracked tooth can occur on the crown and extend into the root developing from occlusal forces or as a direct result of weakened tooth structure. Variable signs and symptoms may exist. Vertical root fracture occurs and originates in the roots with variable signs and symptoms caused by wedging forces within the roots (such as root canal obturation or posts). Split tooth is a fracture through the crown and root developing from damaging occlusal forces or weakened tooth structure resulting in separation of the tooth into two segments. The tooth is typically painful on mastication [48, 50, 51, 58–60].
Radiographic determination of the presence and extent of cracks or even vertical root fractures cannot be consistently determined using conventional plain film radiography or with the recent advancements in cone-beam volumetric tomography (CBVT) scans [61–63]. Axial slices were more accurate in the detection of vertical root fractures compared to sagittal and coronal slices (see Fig. 16.3). The obvious sign of root fracture is the radiographic appearance of root segment separation. Strong indications in ascertaining the presence of a vertical root fracture include the presence of a ‘halo’ appearance, which is a combined peri-apical and perilateral radiolucency in one or both sides of the root, lateral periodontal radiolucency along the side of the root or angular radiolucency from the crestal bone terminating along the root side. In mandibular molars, radiolucency in the furcation area can also be observed coupled with the types of radiolucencies described above [58, 64, 65].
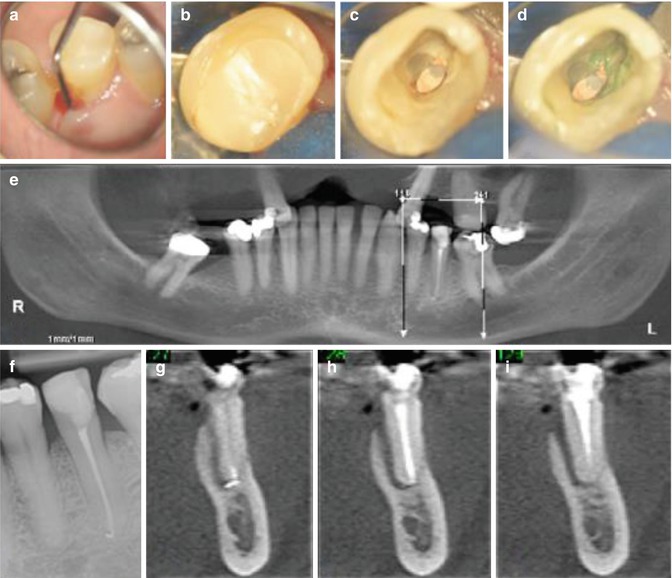

Fig. 16.3
Clinical photographs and radiographs demonstrating difficulties when diagnosing vertical root fracture. Note (a) preoperative view of tooth 35 which had undergone previous root canal treatment. Clinical examination revealed (a) a narrow 10 mm + probing profile on the lingual aspect. (b–d) Following removal of the coronal restoration, no obvious crack line was evident internally despite staining with methylene blue dye. The radiographic appearance using conventional digital radiography revealed no unusual peri-radicular pathology in relation to this tooth (f). A cone-beam CT scan (e, g–i) was arranged confirming an extensive J-shaped peri-radicular radiolucency indicative of a probable vertical root fracture. The patient was referred for extraction and alternative prosthodontic replacement of this tooth
Management options for cracked teeth have the most variation due to the difficulty of accurately predicting the true extension and depth of the crack line. Treatment options for cracked teeth with vital pulps include cuspal coverage crown reinforcement [66] or bonded restorations [59, 67]. If the fracture line has progressed further down the root resulting in bacterial ingress into the pulp chamber, then the pulp may become nonvital requiring endodontic treatment [48, 50, 59, 68]. The prognosis for both vertical root fractures and split teeth is poor, and extraction should be considered [58–60].
Root resection is the surgical procedure by which one or more of the roots of a multi-rooted tooth are removed at the level of the furcation whilst the crown and remaining roots are left in function [69]. Endodontic indications include root fracture or perforation, external root resorption, failed root canal treatment, root caries or endodontic–periodontal combined lesions. Periodontal indications include moderate to advanced furcation involvement, severe bone loss affecting one or more roots, severe recession or dehiscence of a root or unfavourable root proximity between adjacent teeth [70]. The prognosis of root resection has been well documented in the literature. Carnevale reported a group of 72 patients with 175 furcated molars, treated with root resection and prosthetic restoration and followed longitudinally for 10 years. At the 10-year examination, the tooth survival rate was 93 % and the prosthetic survival rate was 96 %. The causes of failure were peri-apical granuloma, secondary decay, reoccurrence of periodontitis and root fracture [71]. Combined endodontic, periodontal and prosthodontic measures to ensure the retention of a tooth or root(s) can be complex, time-consuming and costly. With the increased use of endo-osseous dental implants to replace failed or failing teeth, the role of a root resection as part of the treatment option has come into question. Nevertheless, when comparing the prognosis of root resection therapy to that of a dental implant, Fugazzatto and colleagues reported a 15-year cumulative success rate of 96.8 % for root resected molars and 97.0 % for molar implants. They concluded that molar root resection therapy and implant therapy had a high degree of functional success [72]. Such outcomes are usually achieved with extremely motivated patients who have been treated by experienced clinicians under ideal conditions.
Endodontic–periodontal lesions commonly affect teeth concurrently by pulpal and periodontal infection leading to pulpal necrosis and attachment loss, respectively. The long-term, successful prognoses for these teeth depend on a number of crucial and key factors including the severity and extent of the initial peri-apical and periodontal infections, the correct treatment planning and decision-making, the skill and experience of the clinician(s) and the motivation of the patient, particularly with long-term periodontal care [73].
16.2 Diagnosis of Endodontic–Periodontal Lesions
The diagnosis of periodontal–endodontic lesions is based on a thorough history correlated with clinical examination and radiographic findings encompassing pulp sensitivity tests, percussion, transillumination, probing pocket depths, mobility assessment and identification of bleeding and/or suppurating pockets.
It is important to determine if the periodontitis is generalised or limited to one site or tooth. The pocket morphology can also provide a clue as to the likely origin of the infection. A narrow periodontal pocket suggests an endodontic cause or tooth fracture. Conversely, pocket formation due to periodontitis is broad at the gingival margin and narrows towards the apex as the periodontal lesion advances. When a narrow pocket is found in a tooth with a vital pulp response, it is important to consider that narrow pockets may also be associated with developmental grooves, fused roots or cervical enamel projections into furcation areas.
Pulp vitality tests can be used although the results need to be interpreted carefully. Sensibility tests determine the response of the nerve but do not evaluate the state of the pulp’s blood supply, which is of greater importance. Furthermore, the results may be inaccurate in multi-rooted teeth where the pulp status may vary between roots. This highlights the importance correlating sensibility tests with the examination and radiographic findings.
Radiographic assessment confirms the provisional diagnosis based on history, examination and special tests. The radiograph will provide information on the location of the lesion (apex, crest or furcation area) and will also determine the presence of factors that may contribute to pulpal death, such as the presence of a deep restoration. Radiographic change at the apex can be a good indicator of an infected pulp, but may not be visible until 2–4 months after pulpal infection. Apart from a peri-apical radiolucency, there may also be signs of a widened periodontal ligament or loss of the lamina dura.
Primary endodontic lesion
A tooth with a necrotic pulp draining through the periodontal ligament into the gingival sulcus is termed chronic apical periodontitis with suppuration. Although this condition mimics a periodontal abscess in reality, pulpal testing will confirm a nonvital tooth. Clinical examination will reveal a narrow pocket as a result of a sinus tract from pulpal origin that opens through the periodontal ligament area. From a diagnostic perspective, the insertion of a gutta-percha point into the sinus tract and radiographic evaluation should confirm the origin of the lesion (Fig. 16.4). When the pocket is examined, it should be narrow, lacking width and isolated. A similar situation may arise if the drainage from a necrotic pulp extends into the furcation area from the lateral canals.
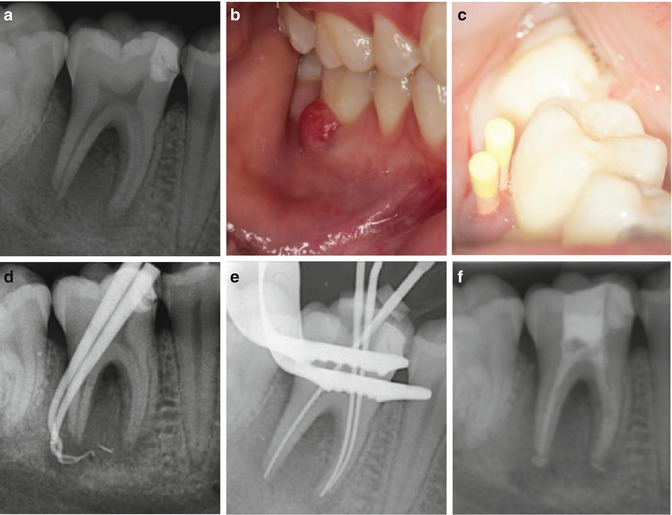

Fig. 16.4
Clinical photographs and radiographs demonstrating tracking of draining sinus and periodontal pocket associated with a necrotic tooth 46. Note (a) preoperative radiograph demonstrating an extensive mesial restoration overlying the mesial pulp horn, (b) a gingival swelling and draining sinus with associated narrow probing profile of 10 mm +, (c) gutta-percha points placed in the overlying sinus and pocket, (d) gutta-percha tracing radiograph confirming suppuration and pocket associated with distal necrotic root of tooth 46, (e) master apical file radiograph following chemo-mechanical preparation and (f) intra-canal calcium hydroxide medicament
Primary endodontic lesions will usually heal following adequate root canal treatment. The sinus tract extending into the gingival sulcus area will disappear following chemo-mechanical instrumentation and intra-canal medication placement. Often the patient can be reviewed 4 weeks later and resolution of the false pocket indicative of diagnosis. Completion of endodontic treatment to an acceptable standard will prevent any further infection.
The astute clinician must also be aware that there are a number of narrow sinus tract type of probing defects associated with a tooth with a vital pulp (see Table 16.2). Pulp testing procedures are critical to accurate diagnosis, and one must bear in mind that the same clinical situations outlined may invariably be associated with a nonvital tooth further confounding an accurate diagnosis.
Table 16.2
Clinical situations resulting in a narrow probing defect associated with a normal vital pulp
|
Situation
|
|
|---|---|
|
Sinus tract draining through the periodontal ligament of a vital tooth
|
The sinus tract may originate from an adjacent nonvital tooth or pulpless tooth several teeth away. Such a sinus tract could also be related to advanced periodontal disease
|
|
Developmental grooves
|
Developmental grooves commonly break down as a narrow sinus tract probing defect. The probing contours may change over time particularly if an acute infection develops
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


