Introduction
The purposes of this study were to longitudinally evaluate the effects of pilot holes on miniscrew implant (MSI) stability and to determine whether the effects can be attributed to the quality or the quantity of bone surrounding the MSI.
Methods
Using a randomized split-mouth design in 6 skeletally mature female foxhound-mix dogs, 17 MSIs (1.6 mm outer diameter) placed with pilot holes (1.1 mm) were compared with 17 identical MSIs placed without pilot holes. Implant stability quotient measurements of MSI stability were taken weekly for 7 weeks. Using microcomputed tomography with an isotropic resolution of 6 μm, bone volume fractions were measured for 3 layers of bone (6-24, 24-42, and 42-60 μm) surrounding the MSIs.
Results
At placement, the MSIs with pilot holes showed significantly ( P <0.05) higher implant stability quotient values than did the MSIs placed without pilot holes (48.3 vs 47.5). Over time, the implant stability quotient values decreased significantly more for the MSIs placed with pilot holes than for those placed without pilot holes. After 7 weeks, the most coronal aspect of the 6- to 24-μm layer of cortical bone and the most coronal aspects of all 3 layers of trabecular bone showed significantly larger bone volume fractions for the MSIs placed without pilot holes than for those placed with pilot holes.
Conclusions
MSIs placed with pilot holes show greater primary stability, but greater decreases in stability over time, due primarily to having less trabecular bone surrounding them.
Highlights
- •
Miniscrew implants (MSIs) in the mandible with or without pilot holes were stable over the short term.
- •
MSIs stability decreased during the first 3 weeks, increased during the next 2 weeks, and then decreased again.
- •
MSIs with pilot holes should be placed with a light constant force to enhance stability.
- •
Seven weeks after placement, MSIs without pilot holes exhibit delayed or diminished healing.
Miniscrew implants (MSIs) provide orthodontists greater anchorage control and orthopedic treatment options. Despite their numerous advantages, the success rates of MSIs have yet to reach those of endosseous implants. Of the various factors proposed to increase MSI stability, the use of pilot holes remains perhaps the most confounded and controversial. Pilot holes decrease insertion torque during initial MSI placement but result in less bone around the MSI after healing.
Pilot holes were first used by surgeons in 1959 to prevent premature failures when placing large pedicle screws for spinal fusion. They were commonly used in the 1980s for orthopedic surgery ; craniofacial surgeons were using pilot holes with screws similar to present-day MSIs. Because of their popularity among surgeons, pilot holes were initially drilled when MSIs were introduced during the early to middle 1990s. However, pilot holes fell out of favor during the late 1990s for their potential disadvantages, including damage to nerves, roots, or tooth germs; thermal necrosis; and drill-bit breakage. Concomitantly, the placement of MSIs without pilot holes became popular with the advent of self-drilling MSIs. Pilot holes came back in favor after 2006 because of concerns about screw breakage during placement and high insertion torque that may be linked to premature MSI failure.
Based on insertion torque, removal torque immediately after insertion, and bone-to-implant contact (BIC) immediately after insertion, pilot holes appear to decrease primary stability. MSIs placed with pilot holes exhibit lower insertion torque than screws placed without pilot holes in both real and synthetic bone. Although pilot holes placed in wood and porcine mandibles had no effect on pullout forces for MSIs, they decreased the pullout forces for MSIs placed in polyvinyl chloride. Hung et al showed that pullout strength decreased as pilot hole size increased in synthetic bone. Studies have also shown that MSIs placed without pilot holes have higher BIC at placement (evaluated by histomorphometry) than do MSIs placed with pilot holes.
Theoretically, lower insertion torque should reduce the strains produced in bone during MSI placement; this might be expected to have a positive effect on secondary stability. Whether MSIs placed with pilot holes exhibit decreased secondary stability remains controversial. Although greater BIC has been reported after 6 months of healing for MSIs placed without than for those placed with pilot holes, no significant difference between MSIs placed with and without pilot holes was reported after 12 weeks of healing.
It is important to determine the effect of pilot holes on the relationship between primary and secondary stability. This relationship remains unclear because it is difficult to evaluate MSI stability longitudinally without destroying the bone-MSI sample. Resonance frequency analysis, a well-established technique for evaluating the longitudinal stability of endosseous implants, is currently the best noninvasive way to monitor implant stability. Resonance frequency analysis produces an electromagnetic signal that excites a metal peg screwed into the implant, and the resonance vibration of the implant is sensed by a transducer in the hand piece. The vibrations are quantified as the implant stability quotient (ISQ), which provides a measure of the implant’s stability, ranging from 1 to 100. Resonance frequency analysis was first used to assess MSI stability in porcine models. More recently, it has been shown that resonance frequency analysis can be used to evaluate MSI stability over time in canine models. Most recently, the use of resonance frequency analysis to measure MSI stability has been validated and applied clinically in humans.
Using the Osstell Mentor (Osstell AB, Gotheburg, Sweden), the objectives of this study were to evaluate the stability over time of MSIs placed with and without pilot holes. To better understand the effects of pilot holes on bone healing, bone surrounding the MSIs was evaluated 3 dimensionally with microcomputed tomography, which is more sensitive than histomorphometry for quantifying experimental effects on bone around MSIs.
Material and methods
Six skeletally mature female foxhound mixes, approximately 2 years of age and weighing between 55 and 65 pounds, were used for this study. Foxhound dogs are an established model for investigating peri-implant osseous dynamics; their large jaws allow for the placement of multiple MSIs. The Institutional Animal Care and Use Committee at Texas A&M University Baylor College of Dentistry in Dallas, Tex, approved the care of the animals and the experimental protocols.
The MSIs used (Neodent, Curitiba, Paraná, Brazil) were self-drilling and made of titanium alloy; the threaded portions were 5 mm long and 1.6 mm wide (outer diameter). The MSIs were fabricated to have threaded SmartPeg (Osstell AB) accepting heads. To prevent tissue overgrowth, each animal had 6 to 8 MSIs placed on the lingual surface of the mandible. MSIs were placed in the furcations and between the teeth, depending on space availability in each dog. Using random assignment, pairs of MSIs were placed in the same location in both sides of the mandible: one with a pilot hole and one without. The Osstell device (Osstell AB) was used to measure implant stability immediately after MSI placement and every week thereafter until the dogs were euthanized. Bony adaptation was evaluated 3 dimensionally with microcomputed tomography and histologically with fluorescence.
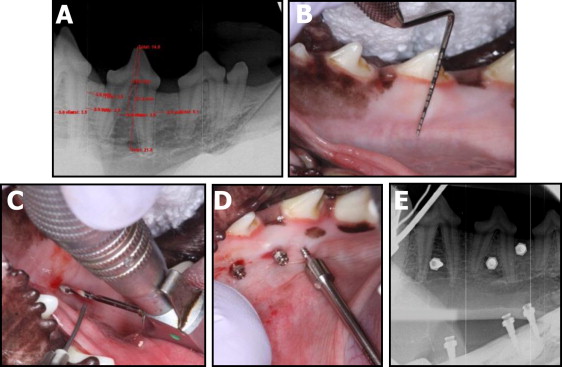
After quarantine and before MSI placement, all animals were sedated with ketamine (2.2 mg/kg intramuscularly) and rompin (0.22 mg/kg intramuscularly) and given a prophylaxis with ultrasonic scaling with chlorhexidine. On the day of MSI placement, periapical radiographs were taken bilaterally and measured to determine screw locations ( Fig 1 , A ). The animals were intubated and maintained with 1% isoflurane with oxygen at 1 L per minute. Radiographic measurements were transferred intraorally via a perio probe ( Fig 1 , B ), and the sites intended for MSI placement—interdental or interradicular—were marked. Local anesthetic (2% lidocaine with 1:100,000 epinephrine) was administered via local infiltration. A 1.1-mm drill bit (Neodent; 3M, St Paul, Minn) was used for the pilot holes. They were drilled at 1600 revolutions per minute all the way through the cortical bone under copious irrigation ( Fig 1 , C ). All MSIs were placed by hand ( Fig 1 , D ), with care taken to ensure that each pair of MSIs was screwed into the bone at the same level. Radiographs were taken again after placement to ensure proper MSI placement ( Fig 1 , E ). Both analgesics (torbugesic 0.2 mg/kg, 2 mg/mL with 1 mL per animal) and antibiotics (penicillin G 60,000 units/kg) were administered. For histologic evaluations of newly calcified bone, calcein was administered intravenously 2 weeks after MSI placement, and tetracycline was administered 5 weeks after placement.
A total of 40 MSIs were placed (all as contralateral pairs of experimental and control), and the experiment lasted for 7 weeks. Thirty-four MSIs were placed at baseline; 2 MSIs (1 experimental, 1 control) were placed at 4 weeks to evaluate healing at its theoretical low point ; 4 more MSIs (2 experimental, 2 control) were placed when the dogs were killed (7 weeks). Inspection of MSIs and tissue conditions, administration of chlorhexidine gel, and Osstell ISQ measurements were performed weekly.
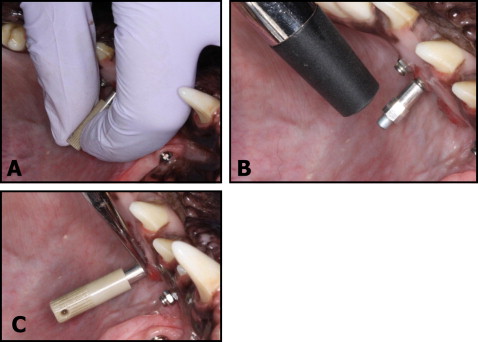
The Osstell Mentor uses an electromagnetic signal to measure the implant’s stability based on how much the SmartPeg vibrates. The Osstell device has been shown to be reliable for measuring MSI stability in vitro and in vivo. The SmartPeg was screwed into each MSI according to the manufacturer’s recommendations ( Fig 2 , A ). Four measurements—2 roughly parallel to the occlusal plane and 2 roughly perpendicular to the occlusal plane—were taken perpendicular to the long axis of the MSI and averaged ( Fig 2 , B ). The MSI was stabilized with a hemostat for SmartPeg insertion and removal ( Fig 2 , C ).
At the completion of the 7-week experimental period, the dogs were euthanized, and the mandibles were disarticulated. Each bone-implant sample was retrieved with a modified 10-mm-diameter trephine bur (ACE Dental Implant System, Brockton, Mass) under copious irrigation. The samples were trephined parallel to the long axis of the implant to reduce scatter during scanning. Care was taken to ensure that all layers of the bone remained undamaged during the retrieval process. The samples were labeled and stored in 70% ethanol under limited lighting conditions.
When needed to ensure their precise fit into the cylindrical (9.8-mm internal diameter) sample holders used for scanning, a wet grindstone was used to smooth the outer edges of the bone-implant specimens. Four to 6 bone-implant specimens were placed in each holder, separated by sponges, and kept hydrated with 70% ethanol. The samples were evaluated using microcomputed tomography (modol 35; Scanco Medical, Bassersdorf, Switzerland) with an isotropic resolution of 6 μm. X-ray energy levels were set to 70 kVp, current to 114 μA, and integration time to 800 ms. A 0.5-mm aluminum filter and a high resolution setting of 1000 projections per 180° were used to ensure the highest quality scans with minimal metal implant artifact. The average scanning time was approximately 3.2 hours per specimen.
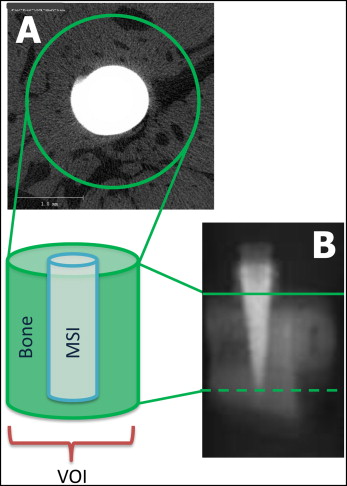
Cortical and trabecular volumes of interest were determined for each specimen. The volumes of interest were horizontally defined by circles drawn around the center of each MSI and perpendicular to the long axis of the MSI ( Fig 3 ). The coronal limit of the cortical volume of interest was visually defined as the first slice showing bone completely surrounding and contacting the MSI. The apical limit of the cortical volume of interest was visually defined as the most apical slice showing no trabecular bone (based on the absence of marrow spaces). The coronal limit of the trabecular volume of interest was defined visually as the most coronal slice with only trabecular bone surrounding the MSI; the apical limit was visually defined at the MSI apex ( Figs 3 and 4 ).
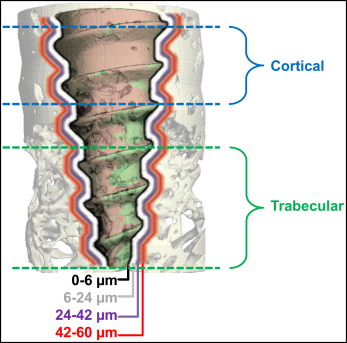
After scanning, a threshold (1 gray scale number, above which all voxels were considered to be bone, and below which all voxels were considered not to be bone) was determined based on multiple comparisons to distinguish the MSI from mineralized bone and background. Scanco Medical proprietary programming scripts with different parameters for BICs were processed for each sample. Separate bone volume to total volume fractions (BV/TV) were calculated for the cortical and trabecular volumes of interest. Based on previously established protocols, BV/TV values of 3 layers of bone surrounding the MSI were calculated. Each layer was 3 voxels (3-dimensional space with an associated gray-scale “brightness” value) thick, extending 6 to 24, 24 to 42, and 42 to 60 μm from the MSI surface ( Fig 4 ). BV/TV values of 0 to 6 μm from the MSI were excluded because of the metallic halation artifact.
Five pairs of bone-MSI specimens were evaluated histologically: 3 placed initially, 1 placed at 4 weeks, and 1 placed at 7 weeks that served as a control. They were dehydrated in graded alcohol over a period of 1 month and soaked in acetone for 3 days, methyl methacrylate monomer number 1 for 7 days, and methyl methacrylate monomer number 2 for 7 days. The samples were then embedded in methyl methacrylate until polymerization was complete. Serial sections of the samples were made. The slice that showed the greatest surface area of the MSI was polished and evaluated. The fluorescent signal was imaged using a fluorescent microscope (Nikon, Melville, NY) and a Photometrics CoolSnap K4 CCD camera (Roper Scientific, Duluth, Ga), and the signal was captured with NIS-Elements software (Nikon). Images were captured for calcein (green) using a filter with an excitation between 395 and 580 nm and an emission between 480 and 520 nm; the images for tetracycline (red) were captured using an excitation filter between 395 and 580 nm and an emission filter between 520 and 570 nm.
Statistical analysis
Because ISQ values at some time points were not normally distributed, they were described with medians and interquartile ranges. Changes in ISQ values over time and differences between sides were evaluated using Wilcoxon signed ranks tests.
To quantify BV/TV from the 360° segmental analysis, the cortical and trabecular layers of bone were evaluated separately. Due to variations in the number of slices between the most coronal and apical aspects of each layer, the slice number was divided by the total number of slices to calculate relative section thicknesses ranging from 0% to 100%.
Multilevel statistical models were used to determine whether changes in BV/TV occurred between the most coronal and apical aspects of each layer within each section. The same models were used to statistically evaluate differences in bone surrounding the MSIs that had been placed without (control) and with (experimental) pilot holes. The models were developed with MLwiN software (Center for Multilevel Modeling, Institute of Education, London, United Kingdom) using iterative generalized least squares. The analyses showed that the repeated measurements of BV/TV were best described by simple linear regression models, with the intercept describing BV/TV at the 0% relative layer thickness (the most coronal slice) and the slope describing the changes in BV/TV for each percent of change of relative section thickness. For these analyses, 3-level models were used with random variations partitioned between specimens, between sides within specimens, and between slices within each side. Separate models were developed for each of the 3 layers of bone in the cortical and trabecular sections.
Multilevel modeling was chosen because (1) it makes no assumptions as to the equality of the intervals used for the independent variable, and (2) it possible to simultaneously determine the appropriate curves to use and to evaluate any group differences.
Results
All MSIs remained in place until the dogs were euthanized; none exhibited mobility. Four (10%) of the MSIs turned slightly (less than half a rotation) during placement or removal of the SmartPeg; none became unstable. The tissues around 11 of the 40 MSIs were inflamed at some point during the 7-week experimental period. This inflammation was usually due to food debris in or around the MSI head and usually resolved quickly, since the MSIs were irrigated and chlorhexidine gel was applied each week.
At placement and 1 week later, the MSIs with pilot holes showed significantly higher ISQ values than did the MSIs placed without pilot holes ( Table I ). The other ISQ values were generally higher for MSIs without pilot holes than for those with pilot holes, but no difference was statistically significant.
| No pilot hole | Pilot hole | Side differences | ||||
|---|---|---|---|---|---|---|
| Median ISQ | Interquartile range | Median ISQ | Interquartile range | Median | P value | |
| T0 | 47.5 | 43.3-48.8 | 48.3 | 47.1-51.8 | −2.344 ∗ | 0.019 ∗ |
| T1 | 44.5 | 41.0-48.9 | 47.5 | 44.4-50.6 | −3.011 ∗ | 0.003 ∗ |
| T2 | 45.0 | 41.3-48.0 | 44.5 | 39.0-47.8 | −0.129 | 0.897 |
| T3 | 43.5 | 38.5-45.0 | 44.0 | 37.8-46.0 | −0.331 | 0.740 |
| T4 | 45.0 | 40.0-48.0 | 44.0 | 37.3-47.5 | −0.190 | 0.850 |
| T5 | 46.0 | 39.5-48.0 | 44.0 | 38.0-49.0 | −0.308 | 0.758 |
| T6 | 45.0 | 38.5-47.5 | 43.5 | 40.5-48.5 | −0.795 | 0.426 |
| T7 | 45.0 | 34.3-46.8 | 41.0 | 38.0-45.5 | −0.783 | 0.434 |
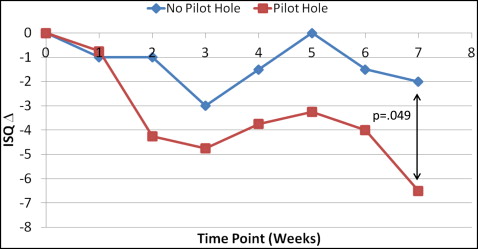
The median changes in ISQ values from baseline showed decreases during the first 3 weeks, increases over the next 2 weeks, and decreases over the last 2 weeks ( Fig 5 ). Although the decreases from baseline were generally greater for the MSIs with pilot holes than for those without pilot holes, only the difference at week 7 was statistically significant.
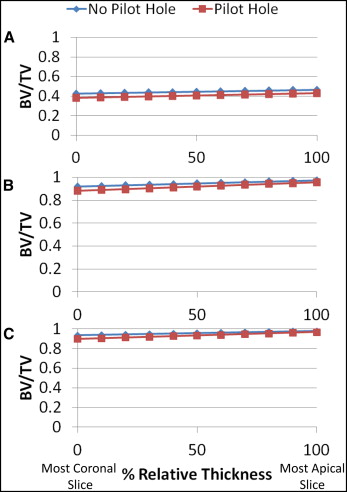
BV/TV increased slightly between the most coronal and apical slices of the cortical sections ( Fig 6 ). There was approximately 4% less cortical bone around MSIs with pilot holes than around those without pilot holes. At the most coronal aspect of the cortical sections, the only statistically significant difference occurred in the 6- to 24-μm layer, which showed 4.3% less bone around MSIs with pilot holes than without pilot holes ( Table II ). MSIs with pilot holes showed greater increases in BV/TV between the most coronal and apical slices than did those without pilot holes, resulting in even smaller differences in BV/TV at the more apical aspects of the cortical sections. Whereas all 3 cortical layers showed greater increases in BV/TV for the MSIs with pilot holes, only the differences in the 24- to 42-μm and the 42- to 60-μm layers were statistically significant.
| Layer (μm) | No pilot hole | Difference (no pilot hole – pilot hole) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Constant | Linear | Constant | Linear | ||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | ||
| Cortical | 6-24 | 0.4258 ∗ | 0.0152 | 0.0405 ∗ | 0.0047 | −0.0432 ∗ | 0.0216 | 0.0059 | 0.0075 |
| 24-42 | 0.9197 ∗ | 0.0147 | 0.0546 ∗ | 0.0055 | −0.0386 | 0.0210 | 0.0217 ∗ | 0.0087 | |
| 42-60 | 0.9355 ∗ | 0.0145 | 0.0441 ∗ | 0.0048 | −0.0353 | 0.0207 | 0.0241 ∗ | 0.0077 | |
| Trabecular | 6-24 | 0.3926 ∗ | 0.0234 | −0.0815 ∗ | 0.0052 | −0.1024 ∗ | 0.0330 | 0.1395 ∗ | 0.0068 |
| 24-42 | 0.9195 ∗ | 0.0324 | −0.2411 ∗ | 0.0068 | −0.1361 ∗ | 0.0434 | 0.2215 ∗ | 0.0090 | |
| 42-60 | 0.9197 ∗ | 0.0334 | −0.2312 ∗ | 0.0066 | −0.1237 ∗ | 0.0428 | 0.2101 ∗ | 0.0088 | |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


