Introduction
Even though the beneficial effects of fluoride on enamel and root caries have been well documented, limited data are available concerning the effect of fluoride on orthodontically induced root resorption and tooth movement. Our objective was to investigate the effect of systemic fluoride administered from birth to 12 weeks on orthodontically induced root resorption and tooth movement in rat molars.
Methods
Fifty male Wistar rats were randomly divided into 5 groups. The negative control group received no sodium fluoride and had no tooth movement. The positive control group received no sodium fluoride but had tooth movement. Three experimental groups received sodium fluoride at 45 ppm from birth to 2, 4, and 12 weeks, respectively. At week 10, a 50-g nickel-titanium coil spring was applied to the maxillary left first molar for 2 weeks. The rats were killed at 12 weeks of age. Movement of the maxillary first molars was measured in relation to the maxillary second molar on digitized cephalometric radiographs. Mesial and distal roots were examined by using scanning electron and 3-dimensional laser microscopes.
Results
Fluoride reduced the depth, volume, and roughness of the resorption craters in the experimental groups. However, the area was similar to that in the positive control group. Regarding the duration of fluoride intake, the longer fluoride was administered via drinking water, the smaller the amount of tooth movement observed.
Conclusions
Fluoride in drinking water from birth reduced the severity of orthodontically induced root resorption, but the amount of tooth movement was also decreased.
Fluoride, the ionic form of the trace element fluorine, is the most electronegative and the 13th most abundant element in the earth’s crust (about 0.07%). The use of fluoride-containing agents has been a successful strategy for the prevention of enamel and root surface caries. Fluoridation of water supplies reduces caries by 50% on average. Because of its high electronegative activity, fluoride reacts promptly to its surroundings. In mineralized tissues, fluoride reacts by hydroxyl group of calcium hydroxyapatite lattice (Ca 10 [PO 4 ] 6 [OH] 2 ) and forms fluoroapatite by replacing it. The structure of the fluoroapatite crystal is larger and less soluble than calcium hydroxyapatite and, therefore, more resistant to demineralization.
Over 99% of the fluoride in the human body is stored in bone, dentin, enamel, and cementum. The concentration of fluoride in mineralized tissues depends on the level of fluoride intake, the duration of exposure, and interrelated factors such as the rate of growth, the stage of tissue development, and the surface area. It has been found that cementum contains the highest concentration of fluoride among the mineralized tissues. Moreover, fluoride concentration of cementum increases with age.
One principal skeletal effect of fluoride is to stimulate the formation of new bone, primarily by an increase in osteoblast numbers in vitro and in vivo. It is believed that sodium fluoride (NaF) directly inhibits the activity of osteoclasts by preventing calcium ion release and directly suppresses osteoclast acidity.
Orthodontically induced root resorption (OIRR) is an inevitable sequela of orthodontic treatment that affects 100% of treated patients. OIRR can be explained as the loss of calcified dental tissues, cementum, and dentin. It occurs during the removal of the hyalinized zone and is caused by localized overcompression of the periodontal ligament. Monocytes, macrophages, osteoclasts, and odontoclasts play major roles in the OIRR process.
The process of root resorption has multiple risk factors; however, it has been suggested that changes in the mineral content of cementum can influence resistance to root resorption. It is believed that increased hardness and density of the dental tissues provides resistance to resorption activity.
Although previous studies investigated the effects of fluoride on tooth movement and root resorption, fluoride was administered only during the experimental tooth movement. We hypothesized that systemic fluoride intake from birth, during tooth development, would have an effect on both cementum and bone, and, as a result, OIRR would be less severe. The aim of this study was to evaluate the effect of fluoride administered from birth for varying lengths of time on root resorption and tooth movement.
Material and methods
The study was approved by the Animal Welfare Committee of Nagasaki University (no. 0603170498) in Japan. Fifty male Wistar rats were randomly divided into 5 groups of 10 rats each. The negative control group received no NaF and had no tooth movement. The positive control group received no NaF but had tooth movement. Three experimental groups received NaF at 45 ppm from birth to 2, 4, and 12 weeks, respectively. The rat pups were separated from their dams at 4 weeks of age. All animals (pups and dams) were fed a standard pellet diet and received water ad libitum. Each rat’s weight was recorded daily after the appliance was placed.
A closed-coil spring of 50 g (Sentalloy, Tomy, Fukushima, Japan) was used to move the maxillary left molar mesially from the 10th to the 12th weeks. The appliance was placed under anesthesia (intraperitoneal injection of pentobarbital) at a dosage of 60 mg per kilogram of body weight. The appliance was placed as previously described ( Fig 1 ). The force magnitude was measured by using a tension gauge (DTN-150, Teclock, Tokyo, Japan) when the appliance was placed and at the end of the experiments.
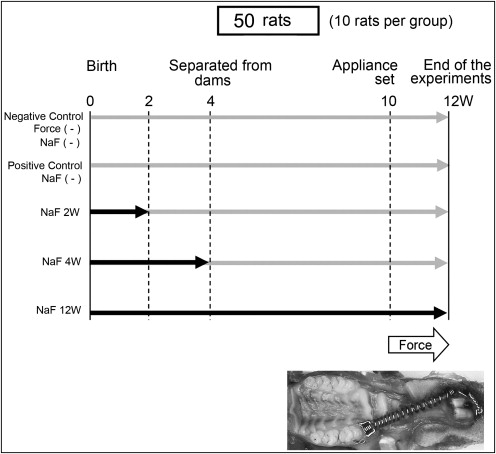
The water was changed daily, and the amount consumed was monitored by measuring the water remaining in the bottle after 24 hours. The fluoridated water was made by dissolving NaF (Wako Pure Chemical Industries, Osaka, Japan) into distilled water to a concentration of 45 ppm, corresponding to about 10 ppm in humans. Tooth movement was measured on digitized lateral cephalometric radiographs, as previously described. The amount of tooth movement at the coronal level was determined by the change in distance between the 2 closest points in the crowns of the first and second molars. At the end of the experiments, the rats were killed by an overdose of carbon dioxide.
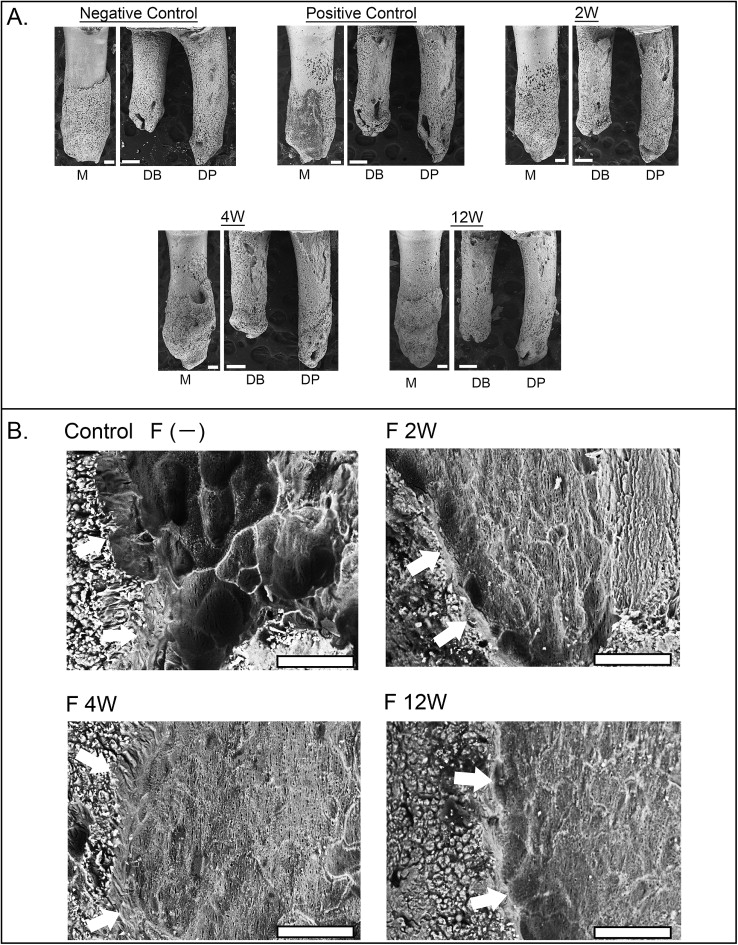
The maxillary left first molar, including its surrounding bone, was cut as a block, followed by delicately removing the alveolar bone to avoid any root surface damage. The molars were submerged in 1% sodium hypochlorite to eliminate any remaining periodontal ligament. The molars were then sectioned buccolingually through the crown near the cementoenamel junction with a thin diamond disk. Root resorption craters on the apical region were not evaluated because of anatomic variations and difficulties in delimiting the craters. The mesial and distal surfaces of the distobuccal, distopalatal, and mesial roots were evaluated with a scanning electron microscope (SEM) (TM-1000, Hitachi, Tokyo, Japan) and a 3-dimensional laser scanning microscope (VK-8500, Keyence, Kyoto, Japan). Since resorption craters on the distal surfaces of the roots were scarcely detectable, they were also excluded from the study. All craters scattered on the cervical and middle thirds of the roots (mesial side) were digitally obtained. Surface area was measured with commercial software (Mimics 11.11, Materialise Software, Leuven, Belgium). The deepest point and the surface roughness of the resorption craters were calculated with the laser microscope program. The crater area value was multiplied by the average depth to give the volume. The same investigator (C.G.) performed all measurements, and every measurement was repeated 3 times. The mean value was used as the final measurement. To assess measurement reproducibility, serial measurements of area, depth, surface roughness, and volume were made 10 times in 1 randomly selected distobuccal root from the experimental control group. The means and standard deviations were 34.1% ± 0.3% for area, 117.5 ± 0.37 μm for depth, 12.9 ± 0.05 × 10 6 μm 3 for volume, and 18.7 ± 0.5 μm for surface roughness.
Statistical analysis
Univariate analysis of variance (ANOVA) followed by Bonferroni adjustments were performed with SPSS software (version 16.0, SPSS, Chicago, Ill).
Results
Fluoride administration in drinking water was well tolerated by all rats. This was confirmed by measuring the amount of water consumed by each rat per day (25-30 mL). The initial weight of the control rats was 272.7 ± 22.7 g, and no statistical difference was found among the fluoride-treated groups. At the end of the experiments, the weights of the controls and the 2-, 4-, and 12-week fluoride-treated rats were 270 ± 27.3, 276.1 ± 11.1, 267 ± 14.7, and 259 ± 11.4 g, respectively. All coil springs were active after 14 days, indicating that force was delivered throughout the experiment. After 2 weeks of force application, the experimental control rats exhibited 0.5 ± 0.08 mm of tooth movement. Fluoride administered for 12 weeks showed the least amount of tooth movement ( Fig 2 , Tables I and II ). There was an inverse relationship between the duration of exposure to fluoride and the amount of tooth movement ( Table III ).
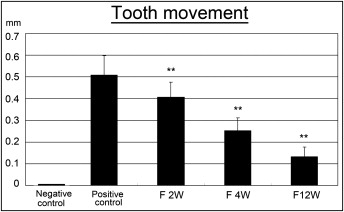
| Mean | ±SD | |
|---|---|---|
| Control | 0.50 | 0.08 |
| F 2W | 0.40 | 0.06 |
| F 4W | 0.25 | 0.05 |
| F 12W | 0.13 | 0.04 |
| Control group (I) | Experimental group (J) | Mean difference (I-J) | SE | Significance † | 95% CI for difference † | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Tooth movement (mm) | ||||||
| F 2W | 0.10 ∗ | 0.02 | 0.00 | 0.03 | 0.17 | |
| F 4W | 0.25 ∗ | 0.02 | 0.00 | 0.19 | 0.32 | |
| F 12W | 0.37 ∗ | 0.02 | 0.00 | 0.30 | 0.45 | |
| r | P value | n | |
|---|---|---|---|
| Tooth movement | −0.910 | 0.000 | 50 |
| Distobuccal roots | |||
| Area (%) | −0.051 | 0.732 | 50 |
| Depth (μm) | −0.937 ∗ | 0.000 | 50 |
| Volume (×10 6 μm 3 ) | −0.869 ∗ | 0.000 | 50 |
| Roughness (μm) | −0.658 ∗ | 0.000 | 50 |
| Distopalatal roots | |||
| Area (%) | −0.138 | 0.356 | 50 |
| Depth (μm) | −0.959 ∗ | 0.000 | 50 |
| Volume (×10 6 μm 3 ) | −0.877 ∗ | 0.000 | 50 |
| Roughness (μm) | −0.884 ∗ | 0.000 | 50 |
| Mesial roots | |||
| Area (%) | −0.165 | 0.268 | 50 |
| Depth (μm) | −0.923 ∗ | 0.000 | 50 |
| Volume (×10 6 μm 3 ) | −0.611 ∗ | 0.000 | 50 |
| Roughness (μm) | −0.646 ∗ | 0.000 | 50 |
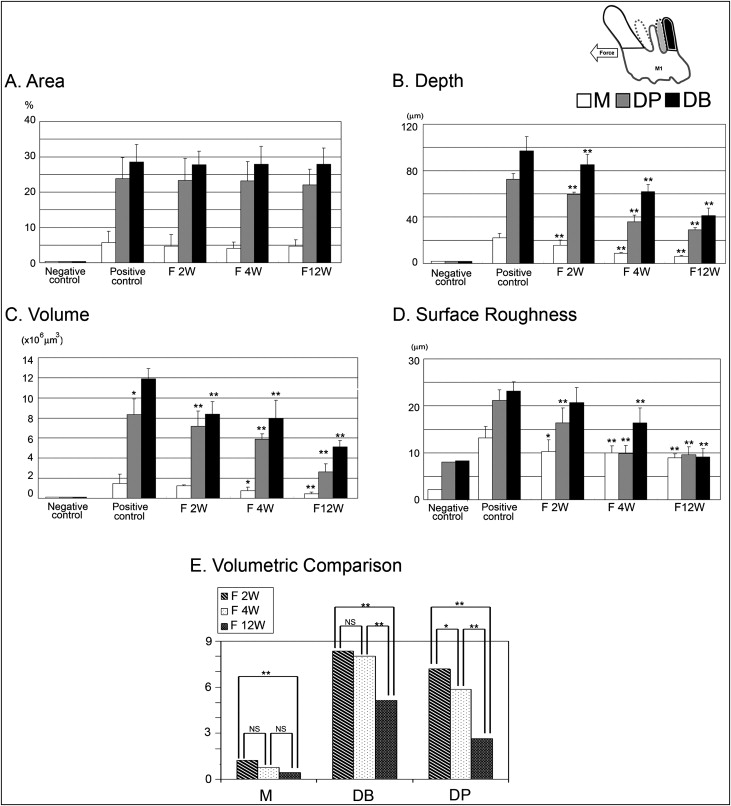
The control roots were covered by undamaged cementum with a characteristic smooth surface. The apical third of the roots was covered with thick cementum with a rough and irregular surface that occasionally contained resorption craters. In all experimental groups, 3 types of resorption craters were found: isolated lacunae, wide shallow resorption pits, and deep resorption craters. Small isolated lacunae were mainly scattered on the mesial roots (cervical half of mesial surfaces). Wide shallow and deep resorption craters were observed on the distal roots covering the cervical and middle portions of the root. The bottom of the root resorption cavities showed extensive, irregular, disorganized, and rough layers with irregular borders ( Fig 3 , A ). Root resorption was quantitatively evaluated by measuring crater area, depth, volume, and surface roughness ( Table IV ). The surface area of the craters showed no significant difference among the groups ( Fig 4 , A ). Interestingly, in the case of the lesions formed without fluoride, the resorption craters were deeper than those formed with fluoride ( Fig 4 , B ). Likewise, the volume of the craters decreased significantly compared with the nonfluoride group ( Fig 4 , C ; Tables V and VI ). When root resorption occurred in the absence of fluoride, the surface of the resorption craters showed deep pits with well-defined margins compared with the fluoride-treated rats ( Figs 3 , B , and 4 , C ). In spite of the difference in the severity of root resorption in the mesial and distal roots, the effect of NaF showed a similar pattern in both roots ( Table IV ).
| Area (%) | Depth (μm) | Volume (×10 6 μm 3 ) | Roughness (μm) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| Distobuccal roots | ||||||||
| Control | 28.5 | 5 | 96.8 | 12.3 | 11.9 | 1 | 23.1 | 1.9 |
| F 2W | 27.8 | 3.8 | 85.15 | 8.7 | 8.4 | 1.2 | 20.7 | 3.1 |
| F 4W | 27.8 | 5.1 | 61.8 | 6.2 | 7.9 | 1.7 | 16.3 | 3.2 |
| F 12W | 27.8 | 4.6 | 41.1 | 6.6 | 5.1 | 0.6 | 9.1 | 1.8 |
| Distopalatal roots | ||||||||
| Control | 23.8 | 5.9 | 72.5 | 4.9 | 8.3 | 1.5 | 21.2 | 2.2 |
| F 2W | 23.2 | 6.3 | 59.7 | 1.8 | 7.2 | 1.5 | 16.3 | 3.2 |
| F 4W | 23.1 | 5.5 | 36.1 | 5.6 | 5.8 | 0.5 | 9.8 | 1.6 |
| F 12W | 22 | 4.4 | 29.17 | 1.7 | 2.7 | 0.8 | 9.6 | 1.6 |
| Mesial roots | ||||||||
| Control | 5.7 | 3.1 | 22.1 | 3.8 | 1.4 | 0.9 | 13.1 | 2.4 |
| F 2W | 4.7 | 3.2 | 5.7 | 4.3 | 1.2 | 0.1 | 10.3 | 2.4 |
| F 4W | 4.1 | 1.7 | 8.6 | 1 | 0.8 | 0.3 | 9.9 | 1.5 |
| F 12W | 4.6 | 1.8 | 6.1 | 0.8 | 0.5 | 0.1 | 8.9 | 0.8 |
| Control group (I) |
Experimental group (J) |
Mean difference (I-J) |
SE | Significance † | 95% CI for difference † | |
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Distobuccal root | ||||||
| Area (%) | ||||||
| F 2W | 0.71 | 2.20 | 1.00 | −5.36 | 6.78 | |
| F 4W | 0.65 | 1.72 | 1.00 | −4.11 | 5.41 | |
| F 12W | 0.63 | 1.93 | 1.00 | −4.7 | 5.96 | |
| Depth (μm) | ||||||
| F 2W | 14.05 | 3.87 | 0.04 | 3.34 | 24.76 | |
| F 4W | 36.86 ∗ | 3.03 | 0.00 | 28.47 | 45.26 | |
| F 12W | 59.11 ∗ | 3.40 | 0.00 | 49.71 | 68.52 | |
| Volume (×10 6 μm 3 ) | ||||||
| F 2W | 3.53 ∗ | 0.56 | 0.00 | 1.96 | 5.10 | |
| F 4W | 3.8 ∗ | 0.44 | 0.00 | 2.57 | 5.04 | |
| F 12W | 6.82 ∗ | 0.49 | 0.00 | 5.44 | 8.20 | |
| Roughness (μm) | ||||||
| F 2W | 2.91 | 1.06 | 0.05 | −0.01 | 5.84 | |
| F 4W | 14.43 ∗ | 0.83 | 0.00 | 12.14 | 16.73 | |
| F 12W | 7.81 ∗ | 0.93 | 0.00 | 5.24 | 10.38 | |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


