Introduction
Our objectives were to study the effect of micro-osteoperforations on the rate of tooth movement and the expression of inflammatory markers.
Methods
Twenty adults with Class II Division 1 malocclusion were divided into control and experimental groups. The control group did not receive micro-osteoperforations, and the experimental group received micro-osteoperforations on 1 side of the maxilla. Both maxillary canines were retracted, and movement was measured after 28 days. The activity of inflammatory markers was measured in gingival crevicular fluid using an antibody-based protein assay. Pain and discomfort were monitored with a numeric rating scale.
Results
Micro-osteoperforations significantly increased the rate of tooth movement by 2.3-fold; this was accompanied by a significant increase in the levels of inflammatory markers. The patients did not report significant pain or discomfort during or after the procedure, or any other complications.
Conclusions
Micro-osteoperforation is an effective, comfortable, and safe procedure to accelerate tooth movement and significantly reduce the duration of orthodontic treatment.
One main issue in orthodontics is prolonged treatment time, leading patients, especially adults, to avoid treatment or seek alternative options such as implants or veneers with less than optimal results. Therefore, the search for methods that decrease the treatment duration without compromising the outcome is a main challenge in orthodontic research. Whereas clinician-optimized treatment through careful diagnosis and treatment planning, as well as patient cooperation, can affect treatment duration, the main factor controlling the rate of the tooth movement is the biologic response to the orthodontic forces. But what controls the biologic response is not clearly understood.
It is generally accepted that the rate of tooth movement is controlled by the rate of bone resorption, which in turn is controlled by osteoclast activity. Therefore, one can assume that the factors recruiting osteoclast precursors from the circulation and stimulating the differentiation of these cells into osteoclasts should play significant roles in tooth movement.
Many studies have reported an increase in the activity of inflammatory markers such as chemokines and cytokines in response to orthodontic forces. Chemokines play an important role in the recruitment of osteoclast precursor cells, and cytokines, directly or indirectly, through the prostaglandin E2 pathway and the RANK/RANKL pathway, lead the differentiation of osteoclasts from their precursors cells into mature osteoclasts. The importance of these factors in controlling the rate of tooth movement can be appreciated in studies where blocking their effect, through medication or genetic manipulation, dramatically reduces the rate of tooth movement. Therefore, it is logical to assume that increasing the expression of these factors should accelerate tooth movement. Our previous animal studies have shown that performing micro-osteoperforations (MOPs) on alveolar bone during orthodontic tooth movement can stimulate the expression of these inflammatory markers, leading to increases in osteoclast activity and the rate of tooth movement.
To investigate whether this phenomenon occurs in humans, we designed a clinical trial to study the rate of canine retraction with or without MOPs. In addition, the effect of MOPs in the stimulation of inflammatory markers was studied at different time points. Finally, the pain and discomfort of the patients during the study were evaluated.
Material and methods
A randomized, single-center, single-blinded study was approved by the institutional review board of New York University. The sample size was selected based on a type I error frequency of 5% and the power of the statistical test set at 90% ( P = 0.9, β = 0.1) using our animal studies as a guide to detect at least a 50% difference in the rate of tooth movement. The inclusion and exclusion criteria are summarized in Table I . Subjects included in the study had fully erupted maxillary canines with a Class II Division 1 malocclusion that required the removal of both maxillary first premolars.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Male and female | Long-term use of antibiotics, phenytoin, cyclosporin, anti-inflammatory drugs, systemic corticosteroids, and calcium channel blockers |
| Age range, 18-45 years | Poor oral hygiene for more than 2 visits |
| Class II Division 1 malocclusion | Extreme skeletal Class II malocclusion, overjet >10 mm, Pg-Nper >18 mm, ANB >7°, SN-GoGn >38° |
| No systemic disease | Systemic disease |
| No radiographic evidence of bone loss | Evidence of bone loss |
| No history of periodontal therapy | Past periodontal disease |
| No current active periodontal disease | Current periodontal disease |
| No smoking | Smoking |
| No gingivitis or untreated caries | Gingivitis and caries |
| Probing depth <4 mm in all teeth | Probing depth >4 mm in any tooth |
| Gingival index ≤1 | |
| Plaque index ≤1 |
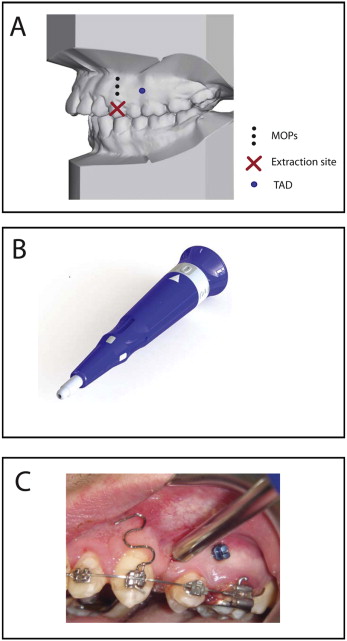
Two orthodontic residents (M.R. and E.K.), trained and calibrated by the principal investigator (M.A.), were responsible for examining the subjects, determining their eligibility, and performing the orthodontic treatment under the supervision of a faculty member who was not the principal investigator. Patients who met the selection criteria and completed an informed consent form were randomly assigned to one of the study groups. The experimental group received MOPs on either the right or left side. MOPs were randomly assigned to the patients’ left or right sides to eliminate the possibility of uneven occlusal forces because of habitual occlusion predominantly on 1 side. The control group received no MOPs. The subjects and the residents administering the treatment were aware of the group assignment and therefore were not blinded. The investigators performing the measurements and data analysis were blinded from the group assignments. Treatment was initiated by bonding fixed appliances in both arches (0.022-in McLaughlin, Bennett, and Trevisi [MBT] prescription) with an auxiliary vertical slot in the maxillary canine brackets (GAC International, Bohemia, NY). Patients were referred for extraction of the maxillary first premolars by the same surgeon to decrease variability. Both the experimental and control groups were leveled and aligned before retraction. At 6 months after the extractions, alginate impressions were taken. Before canine retraction, a periapical x-ray was taken to evaluate the canine root and estimate the center of resistance based on root length. Canine retraction was achieved using calibrated 100-g nickel-titanium closing-coil springs (GAC International) connected from a temporary anchorage device to a power arm on the canine bracket that allowed application of the force closer to the center of resistance of the tooth. At each visit, the force produced by the coil was checked, and the appliances were monitored for any deformation or change in position because of chewing. Load deflection analysis for the 100-g spring showed that the force level remained relatively constant for decreases of 0.5 to 1.5 mm in the length of the spring after initial activation (data not shown). Three MOPs were performed (in the left or right side) distal to the canines and before the retraction ( Fig 1 , A ) using a disposable MOP device designed for this purpose by PROPEL Orthodontics (Ossining, NY) ( Fig 1 , B ). Both temporary anchorage device delivery and MOPs were performed under local anesthesia (2% lidocaine with 1:100,000 epinephrine). No flap was made, and no pain or antibiotic medication was prescribed. The timetable of events is summarized in Table II . After 4 weeks of canine retraction, impressions were taken again, and the study was concluded. Patients continued treatment in the Department of Orthodontics at New York University, and routine final records were taken at the end of treatment.
| Groups | Start time (mo) | Ortho | Ortho + MOPs |
|---|---|---|---|
| Extraction of maxillary first premolars | 0 | ✔ | ✔ |
| Leveling to stage of 16 × 22-in stainless steel | 0-6 | ✔ | ✔ |
| Placement of temporary anchorage devices | 6 | ✔ | ✔ |
| MOP | 6 | ✔ | |
| Canine retraction | 6 | ✔ | ✔ |
| Monitoring OH | 0-7 | ✔ | ✔ |
| Monitoring TM | 6-7 | ✔ | ✔ |
Gingival crevicular fluid (GCF) samples were collected from each subject to evaluate the level of inflammatory response. GCF was collected before orthodontic treatment, immediately before the start of canine retraction, and at each subsequent visit, between 10 am and 12 noon. These samples were taken from the distobuccal crevices of the maxillary canine. If present, supragingival plaque was removed, and cotton rolls were used to isolate the regions before GCF samples were collected with filter-paper strips (Oraflow, Smithtown, NY) inserted 1 mm below the gingival margin into the distobuccal crevices of the canine for 10 seconds. Sample volume was assessed with Periotron 8000 (Oraflow) according to the manufacturer’s instructions. An estimated volume of 0.6 to 1.2 μL of GCF was collected and diluted to obtain 50 to 100 μL of sample, required for analysis, using a glass slide-based protein array. Cytokine levels were measured using a custom protein array for the following cytokines: CCL-2 (MCP1), CCL-3, CCL-5 (RANTES), IL-8 (CXCL8), IL-1α, IL-1β, IL-6, and TNF-α (Raybiotech, Norcross, Ga) according to the manufacturer’s instructions.
Alginate impressions were taken at the beginning of the study, immediately before canine retraction, and 28 days after canine retraction began to monitor the rate of tooth movement. The impressions were immediately poured up with plaster (calcium sulfate). The casts were labeled with the patient’s number and date and stored. Vertical lines were drawn on the cast over the palatal surface of the canine from the middle of the incisal edge to the middle of the cervical line. The distance between the canine and the lateral incisor was assessed before and after canine retraction at 3 points: incisal, middle, and cervical thirds of the crowns. All cast measurements were made using an electric digital caliper (Orthopli Corp, Philadelphia, Pa) with an accuracy of 0.01 mm. Both intraobserver and interobserver errors were evaluated. For the evaluation of the intraobserver error, 10 models were measured twice at least 2 weeks later. For the interobserver error, a second investigator (S.A.) measured the same set of models twice, and the mean values of the 2 measurements by each investigator were compared. The random and systematic errors were calculated using a formula described by Dahlberg and Houston. Both the random and systematic errors were found to be small and insignificant. Random errors were 0.026 mm for the intraobserver evaluation and 0.034 mm for the interobserver evaluation. Systematic errors were 0.025 mm for the intraobserver evaluation and 0.033 mm for the interobserver evaluation ( P <0.001).
The participants were asked to assess their level of discomfort on the day of appliance placement, the day of canine retraction, and subsequently at 24 hours, 7 days, and 28 days after canine retraction with a numeric rating scale, a high reliability tool comparable with a visual analog scale. The patients were instructed to choose a number (from 0 to 10) that best described their pain: 0 would mean “no pain” and 10 would mean “worst possible pain.”
Statistical analysis
Comparisons between groups were assessed by analysis of variance (ANOVA). Pairwise multiple comparison analysis was performed with the Tukey post hoc test. In some experiments, paired and unpaired t tests were used to compare the 2 groups. Two-tailed P values were calculated, and P <0.05 was set as the level of statistical significance.
Statistical analysis
Comparisons between groups were assessed by analysis of variance (ANOVA). Pairwise multiple comparison analysis was performed with the Tukey post hoc test. In some experiments, paired and unpaired t tests were used to compare the 2 groups. Two-tailed P values were calculated, and P <0.05 was set as the level of statistical significance.
Results
Twenty patients were recruited and completed the study with no loss to follow-up. The subjects were selected from patients that came to the Department of Orthodontics at New York University for comprehensive orthodontic treatment between September 2009 and May 2012. Their age range was 19.5 to 33.1 years, with mean ages of 24.7 years for the control group and 26.8 years for the experimental group. The patients were divided randomly into 2 groups with similar severities of malocclusion ( P >0.05) ( Table III ). The control group had 3 men and 7 women, and the experimental group included 5 men and 5 women. All patients maintained good oral hygiene throughout the study and took no additional medications, including analgesics.
| Ortho | Ortho + MOPs | Significance | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| SNA (°) | 81.34 | 2.76 | 82.21 | 3.04 | NS |
| SNB (°) | 76.06 | 3.12 | 77.49 | 3.48 | NS |
| ANB (°) | 5.48 | 1.85 | 5.02 | 1.68 | NS |
| GoGn-SN (°) | 28.63 | 3.79 | 29.19 | 4.12 | NS |
| PP-MP (°) | 26.61 | 3.42 | 27.23 | 3.11 | NS |
| U1-SN (°) | 108.49 | 5.31 | 107.82 | 4.77 | NS |
| IMPA (°) | 98.14 | 6.61 | 96.91 | 5.93 | NS |
| Overjet (mm) | 5.77 | 1.48 | 5.26 | 1.67 | NS |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


