Fig. 6.1
This schematic drawing shows the relationship between tooth development and pulpal innervation. At early stages, nerve fibers are located below the dental lamina. Axons then form a plexus underneath the tooth organ and innervate the dental follicle but do not enter the dental papilla. Later, when the formation of mineralized tissue is already initiated, nerve fibers invade the tooth pulp, apparently as a result of a shift from secretion of pulpal neurorepelling to pulpal neurotrophic factors (Used with permission of Elsevier from Fried et al. [16])
As seen from this discussion, a shift in expression from neurorepulsive to neuroattractive dental papilla/pulpal factors apparently takes place during odontogenesis. In tissue culture, late embryonic or early postnatal dental mesenchyme strongly attracts TG neurites [19]. The main molecular candidate for this effect is nerve growth factor (NGF). NGF in the developing tooth pulp has been demonstrated with a variety of methods [22–24]. In support of this, mutant mice which lack the high-affinity NGF receptor trkA do not develop a pulpal nerve supply [25]. In addition, glial cell line-derived neurotrophic factor (GDNF) and its receptor GFR-α1 mRNAs are expressed in patterns that suggest that GDNF contributes to the establishment of pulpal innervation [24, 26, 27]. However, in vitro, neutralizing antibodies against NGF, brain-derived neurotrophic factor (BDNF), and GDNF applied to cocultures of pulpal and TG explants do not fully block neurite outgrowth. This could be due to growth-stimulating activities of other GDNF-related factors such as neurturin (NRTN), artemin (ARTN), and/or persephin (PSPN), which are expressed in pulpal mesenchymal cells [28]. It may also be explained by effects from other hitherto largely unexamined pulpal neurotrophic factors, e.g., neuregulins [29].
Once having entered the dental pulp, it is likely that local extracellular matrix (ECM) proteins help guide and promote the growth of axons toward their final targets. Among them, laminins, a group of heterotrimeric αβγ proteins, display a clear-cut specificity in this zone. Pulpal nerves seem to use defined laminin substrates for growth and likely also nerve terminal integrity. Tooth pulp nerves express the laminin chains α2, α4, β1, and γ1, as reported for other peripheral nerves. Larger, but not smaller, nerve fascicles also express α5 [30]. In addition, and unexpectedly, laminin α1 chain immunoreactivity is present in tooth pulp nerve bundles. Nerve trunks display marked immunoreactivity for laminin integrin receptors INTα3, INTα6, INTβ1, and INTβ4 chains. Importantly, laminins 211 (α2β1γ1) and 411 (α4β1γ1) are synthesized and secreted from pulpal fibroblasts and could potentially represent important substrates for pulpal nerve fibers. However, when TG neurons were cultured on isolated laminin-211 or laminin-411 surfaces, only 411 promoted neurite outgrowth. Conversely, 211 exerted minimal, if any, neuritogenic activity and seems rather to be involved in mineralization [31]. Thus, in the tooth pulp stroma, laminin-411 may promote the migration of nerves during development and/or regeneration after injury. Another ECM glycoprotein, reelin, which is important for axon development in the central nervous system, is strongly expressed in fully differentiated human odontoblasts. In vitro cocultures with rat TG neurons have indicated that neurites contact odontoblasts at sites of reelin expression. Consequently, since reelin receptors ApoER-2, VLDLR, CNR, and Disabled-1 are expressed in the trigeminal ganglion, it has been suggested that reelin might be an ECM molecule that is involved in the terminal innervation of the dentin-pulp complex [32]. Other nervous system-related signaling molecules such as glutamic acid, phosphatidylcholine, phosphatidylserine, and phosphatidylinositol are present in the mineralized matrix of the peritubular dentin that encapsulates odontoblast processes [33], where they may interact with axons.
In diphyodont species, the primary dentition is eventually replaced by the permanent dentition. The developmental anatomy of the intradental axons is similar in primary and permanent teeth, although the formation of a sensory innervation is more rapid in deciduous than in permanent teeth [34].
6.3 The Structure of Pulpal Axons
When mature, the innervation of primary teeth is structurally identical to that of permanent teeth, although axon numbers are smaller due to size differences [1]. Within the root pulp of permanent teeth in experimental animals and humans, ~70–90 % of axons are unmyelinated, and most of the remainder seem to be Aδ-fibers [1, 23, 34–36]. This is in agreement with the classical concept of nociceptors and has appeared obvious since pain is the predominant if not the only experience that can be evoked when pulpal nerves are excited. However, the parent axons of most pulp afferents are myelinated and have larger diameters, usually in the Aβ-fibers range [36]. They often have rapid extradental conduction velocities as found in large-diameter fibers, for example, in the cat reaching up to almost 60 m−s, while Aδ axons usually conduct in the order of 25 m−s [37]. Their trigeminal cell bodies are of medium or large sizes and have a number of cytochemical characteristics that are specific for the category of primary sensory neurons usually associated with low-threshold mechanoreceptors (LTMs) (see [38]). These observations suggest that a very large number of pulpal axons are end branches of larger or much larger parent axons that branch, taper, and lose their myelin sheaths. Thus, in the rat, EM analysis has shown that whereas 95.6 % of the parent nerve fibers innervating the dental pulp are myelinated, a minority of all axons in the apical part of the radicular pulp have myelin coverings [36]. Further, within the tooth, the unmyelinated axons show immunoreactivity to specific neurofilament antibodies that are conventional markers for myelinated, medium-sized, and large primary sensory neurons [39, 40] (Figs. 6.2a–c and 6.3a, b). Nonetheless, there is no reason to doubt that some unmyelinated pulpal axons are “true” C-fibers and belong to a restricted proportion of pulp-innervating trigeminal ganglion neurons that are small sized and express heat-sensitive TRPV1 and cold-sensitive TRPA1 receptors [41]. These nerve fibers likely terminate in the coronal pulp and convey thermo-induced pain sensations. Similarly, some thinly myelinated pulpal fibers are most probably genuine Aδs with properties and cell soma sizes that are typical for this category of primary sensory neurons.
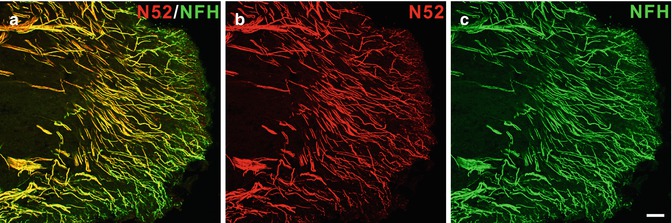
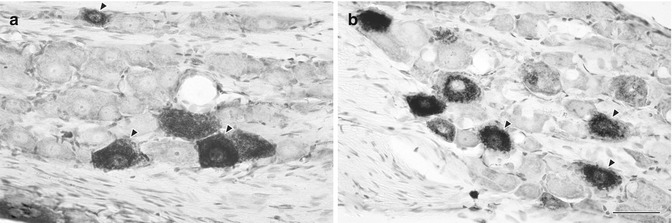

Fig. 6.2
(a–c) Neurofilament 200 kDa expression is prominent in the human dental pulp. Confocal micrographs showing nerve fibers identified by two different neurofilament 200 kDa antibodies [b N52-mouse monoclonal; c neurofilament heavy (NFH)-chicken monoclonal] in the pulp horn of a normal human dental pulp. The overlapping of the N52 and NFH immunoreactivity appears yellow in the merged image (a). Scale bar, 50 μm (Used with permission from Henry et al. [39])

Fig. 6.3
(a, b) Light micrographs showing HRP-labeled somata in the TG that innervate the upper molar (a) and lower incisor (b) pulp. Both large- and medium-sized neurons were frequently labeled. The arrowheads indicate labeled cells with clear nucleoli, selected for measurements of cross-sectional area. Scale bar = 50 μm (Used with permission of Elsevier from Paik et al. [36])
A subset of intradental sensory nerves is involved in the local control of blood flow. By virtue of their neuropeptide content, these afferent fibers cause vasodilation and inhibit sympathetic vasoconstriction in response to painful stimulation of the tooth [42].
As axons traverse the radicular canal to reach the coronal regions of the pulp, they give off a few collaterals, taper, and those that still are myelinated have progressively thinner and shorter internodes [1]. Up to 90 % of the myelinated axons lose their myelin within the short intradental course from the radicular to the coronal pulp [36, 40]. In the pulpal horn, there is an extensive axonal arborization. The sensitivity of the tooth is also most intense here and then gradually declines in parallel with a decrease in nerve fiber density at the pulp-dentin border toward the crown-root transition [43]. Many axons terminate below or in the odontoblast layer region. Near the terminals, they lose their Schwann cell ensheathment altogether, assuming intimate relationships with odontoblasts as well as with specific sub- and periodontoblastic cells with features similar to central nervous system glia. These cells are associated with the local microcirculation in what seems to be analogous to a blood-barrier system [6]. Some axon terminals proceed beyond this site and continue along odontoblast processes into dentinal tubules to innervate the inner segment (0.1 mm) of the dentin. A single intrapulpal axon might branch and innervate more than 100 dentinal tubules ([44]; for further references, see [1, 34]). The fact that mature odontoblast processes and associated nerve fibers are embedded in mineralized dentin limits their accessibility for structural as well as functional studies. Consequently, many aspects of the complex nerve-odontoblast architecture and possible interactions remain obscure. A number of electron microscopical studies on odontoblast-axon relationships have yielded inconclusive results (for references, see [45]). This is probably to some extent caused by inadequate preservation techniques, which fail to maintain the native morphology. Thus, samples from the pulp-dentin junction usually have to be decalcified, which removes the peritubular dentin and distorts estimations of tubule and periodontoblastic size and content [46]. Even more important though is a lack of reliable markers in existing reports to determine the identity of cellular elements in ultrathin sections. In what seems to be a singular exception, an anterogradely transported neuronal tracer was used to examine odontoblast-predentin-dentin innervation. Here, it was concluded that clear-cut ultrastructural signs of synaptic formations were absent from this region [47].
6.4 Neuropeptides in Pulpal Afferents
The neurons innervating the dental pulp express numerous biologically active neuropeptides that are released from both the peripheral terminal of the neurons (within the pulp) and the central terminal located within the trigeminal nuclear complex in the medulla. Some of the neuropeptides identified in pulpal afferents include substance P, calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY), and somatostatin. In the periphery, these neuropeptides have multiple varied effects including regulating blood flow, recruitment and modulation of activity of immune cells, and finally proliferation of and secretion of bioactive molecules from pulpal fibroblasts [48, 49]. Sensory neurons themselves express receptors for neuropeptides; thus, peripherally and centrally released neuropeptides bind to membrane-bound neuronal receptors, either increasing or decreasing neuronal activity, and thus modulating inflammatory pain states.
Small diameter C-fiber neurons expressing the neuropeptides CGRP and substance P represent an anatomically and functionally distinct class of sensory neurons than those without peptides, which typically express a different set of markers including the IB4-lectin binding site, the purinergic P2X3 receptor, and the Mrgprd receptor [50, 51]. The peptidergic and non-peptidergic C-fibers are responsive to different growth factors with the non-peptidergic fibers responding to GDNF and the peptidergic to NGF via the trkA receptor. Interestingly, the dental pulp appears to mostly lack the non-peptidergic C-fiber population, but is well populated by the peptidergic fiber types, both with and without myelin. Other “deep” tissues, including the knee joint and intestines, also have very low levels or even no innervation by non-peptidergic neurons, in contrast to superficial tissues such as the skin in which these fibers are plentiful [52, 53]. The biological consequence of this unique property of neurons innervating the dental pulp is not fully understood, but it could be relevant to the quality and persistence of pain states produced in the setting of injury to pulpal tissues [54, 55].
The neurotransmitter CGRP is expressed in many neurons that innervate the dental pulp, more so than other functionally important neurotransmitters like substance P. Further, the CGRP-expressing pulpal afferents are likely anatomically and functionally unique relative CGRP-expressing afferents innervating other tissues [56–59]. The expression of CGRP in pulpal afferents is dynamic, with increased expression observed after pulpal injury [60–62]. Anatomical studies demonstrate that CGRP-expressing axons will sprout adjacent to an area of a dentinal damage and this sprouting precedes the observation of reparative dentin deposition [63] (Fig. 6.4a–d). After artificial mechanical exposure of the dental pulp to the oral environment, sprouting of CGRP-expressing axons is observed in the remaining vital pulp tissues, adjacent to abscesses where no vital tissue is found [64]. Although in these experiments CGRP was primarily used as an anatomical marker of pulpal axons, there is good evidence that CGRP mediates numerous effects on resident cells of the pulp, supporting the hypothesis that CGRP release from sensory neurons is an important component of healing and repair processes. The function of CGRP has been more thoroughly studied in the context of bone physiology, where it plays an important role in bone healing and remodeling, in part by inducing osteoblast proliferation and differentiation of stem cells into osteoblasts [65–67]. Similarly, in the dental pulp, CGRP can promote the proliferation of fibroblasts, causing BMP-2 production, and thus could potentially stimulate dentin formation [68–71]. Further in vivo experiments are needed to determine if this is a mechanism that can be utilized to promote dentin bridge formation and pulpal healing after injury.
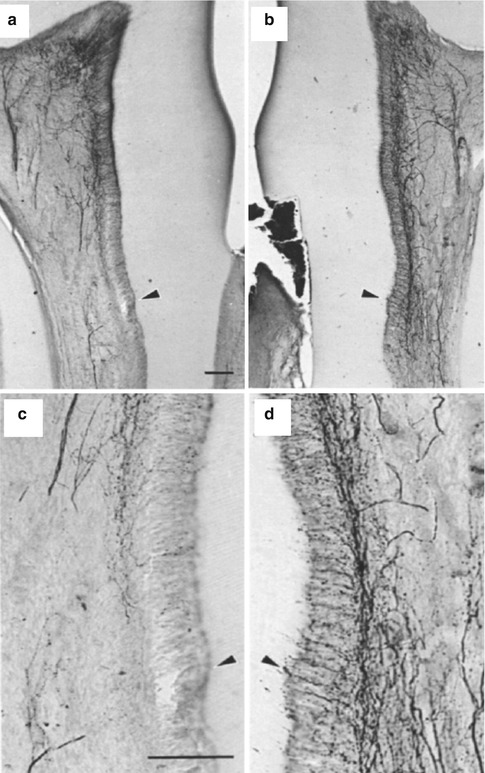
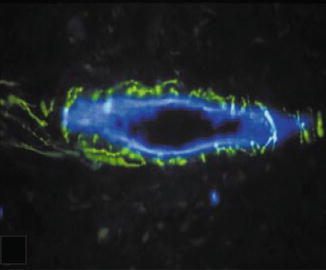
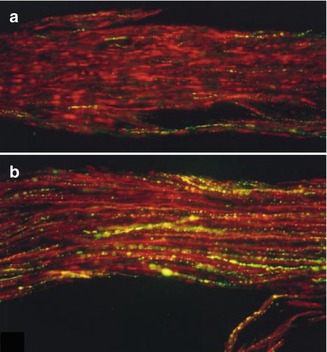

Fig. 6.4
(a–d) Sprouting of CGRP fibers in response to dentinal injury (Used with permission of Elsevier from Taylor et al. [63])

Fig. 6.5
Substance P-expressing fibers (green) forming a plexus around a blood vessel (blue) (Used with permission of John Wiley and Sons from Rodd and Boissonade [75])

Fig. 6.6
(a, b) Substance P upregulated in carious human teeth (Used with permission of John Wiley and Sons from Rodd and Boissonade [84])
In addition to influencing healing and repair via fibroblasts, CGRP release from sensory neurons mediates several aspects of inflammatory processes. CGRP is a potent vasodilator and also causes plasma extravasation [72]. In fact, activation of sensory neurons in the pulp produces an overall vasodilatory effect and increases vascular permeability [73]. In contrast, activation of sympathetic neurons produces vasoconstriction, mediated by both monoamine sympathetic neurotransmitters as well as the peptide NPY [74]. CGRP, substance P, and sympathetic NPY-expressing nerve fibers are found in abundance in close approximation to arterioles [75] (Fig. 6.5). Like CGRP, substance P also causes vasodilation, and the magnitude of their individual vasodilatory effects is augmented when they are co-administered [76].
CGRP and substance P also produce several effects on the immune system. Although there are contradictory findings, the effects of CGRP are found to generally inhibit the immune responses, while substance P is an immune system stimulant [77, 78]. However, in vivo experiments in rats show that denervating the pulp results in reduced immune cell recruitment in response to experimental cavity preparation, suggesting an overall immunostimulatory effect of sensory neuron activation. Both CGRP and substance P cause cytokine release from pulpal fibroblasts [79]. Relevant to inflammatory mechanisms in the dental pulp, CGRP was recently shown to inhibit the release of bacterially stimulated TNF-α release from macrophages, and reduce lymphadenopathy in vivo, after acute bacterial exposure [80].
The immunomodulatory mechanisms of neuropeptides released from dental pulp afferents are complex, and many questions regarding these processes remain. The more we learn about inflammation, the more difficult it is to interpret findings relating to very specific immunomodulatory effects on overall disease processes. From a high level perspective, it’s important to recognize that pulpal sensory neurons are a critical player in the defense mechanisms of the pulp, as pulpal necrosis proceeds more rapidly in denervated teeth that receive a pulp exposure, than in teeth with intact innervation [81]. As this protective effect is likely related to neurosecretions, manipulation of neuropeptide signaling represents an important potential point of therapeutic intervention in the inflamed pulp. Currently, the options for pulpal therapeutic interventions are expanding to include the promotion of biological repair and regenerative processes; thus, a fundamental understanding of the role of neuropeptides in these processes is needed.
The receptors for neuropeptides are found on peripheral sensory neurons, in the trigeminal nucleus, where processing of sensory signaling occurs, as well as other more rostral neuronal structures involved in pain/sensory perception. Endogenous release of neuropeptides can thus modulate sensory neuron activity and pain. Increased levels of neuropeptides, including CGRP, substance P, and NKA, are found in pulps from carious teeth versus non-carious teeth [56]. However, only substance P expression levels were found to be elevated in symptomatic versus non-symptomatic pulps and as well as elevated in pulpal tissues of patients with irreversible pulpitis [82, 83, 84] (Fig. 6.6a, b). Multiple preclinical studies have supported a role for substance P, via the NK1 receptor, to be an important mechanism for maintaining inflammatory and neuropathic pain states. However, an NK1 antagonist was not successful in demonstrating pain relief in clinical studies [85]. On the other hand, CGRP antagonists have demonstrated clinical efficacy in treating migraine pain [86]. Preclinical studies using animal models of pain have also suggested that CGRP receptors have value as a therapeutic target for neuropathic pain. Interestingly, there may be some specificity toward the trigeminal system for the anti-hyperalgesic effects of CGRP antagonist after nerve injury [87]. NPY was shown to produce anti-hyperalgesic effects via the Y1 receptor in animal models in the pulpal tissues as well as in the spinal system [88]. NPY is highly expressed in the spinal cord and trigeminal nucleus and appears to be an important component of endogenous pain relief [89]. In sum, the receptors for neuropeptides expressed in afferents innervating dental pulp are attractive targets for manipulating pain of pulpal origin.
6.5 TRP Channels
Our current understanding of how peripheral neurons detect and transmit thermal, mechanical, and chemical stimuli is greatly influenced by the characterization of a family of cation-permeable channels, termed the transient receptor potential channels or TRPs [90]. The molecular basis for the specificity of populations of peripheral neurons to detect distinct stimuli (e.g., noxious cold or low pH) can be attributed, in part, to their expression of TRP receptors. Interestingly, the expression of TRPs and other sensory receptors differs by the target tissue being innervated; thus, tissues with unique sensory capacity, such as dental pulp, likely demonstrate unique expression of sensory receptors including TRPs [91].
The most studied TRP channel to date is the TRPV1 receptor. It was the first cloned and is notable for being activated by heat in the noxious range, low pH, and capsaicin, the pungent chemical found in chili peppers that causes a warm or burning sensation when ingested [92, 93]. Interestingly, this channel appears to be underrepresented in neurons innervating the dental pulp relative to its expression in other tissues innervated by trigeminal nerves, including the skin and periodontal tissues [41, 94, 95]. As TRPV1 is required for normal heat detection, the underrepresentation of TRPV1 in dental pulp afferents may be one reason why heat is an unreliable stimulus to evaluate pulpal vitality in a clinical setting [96]. Although TRPV1 may not play an important role in sensation in normal pulp, it is very likely involved in pulpal pain in the setting of inflammation, as the TRPV1 receptor is an important site for the integration of signaling pathways from several inflammatory mediators. Also, TRPV1 appears to be upregulated in inflamed human dental pulp [97, 98]. Finally, as capsaicin, a specific agonist for TRPV1, can stimulate the release of neurotransmitters such as CGRP from rodent and human dental pulp, the TRPV1 receptor is clearly functional in the dental pulp [99, 100].
It is of interest that the TRPV2 receptor, which, like TRPV1, was originally described as heat responsive, is highly expressed in the neurons innervating the dental pulp [41, 94, 95] (Fig. 6.7a–d). The neuronal population expressing TRPV2 does not overlap with those neurons expressing TRPV1. The neurons expressing TRPV2 are larger in diameter and myelinated and thus more likely to be low-threshold mechanosensitive neurons than classical nociceptors [43]. Although the TRPV2 receptor was originally described as heat responsive, this characteristic has only been demonstrated in vitro, and further studies suggest the TRPV2 receptor is likely not involved in thermodetection. However, TRPV2 is clearly expressed on both high-threshold and low-threshold mechanosensitive fibers [101, 102]. Whether TRPV2 is a marker for this class of sensory neurons or is functionally involved in transducing mechanosensation has yet to be clearly demonstrated.
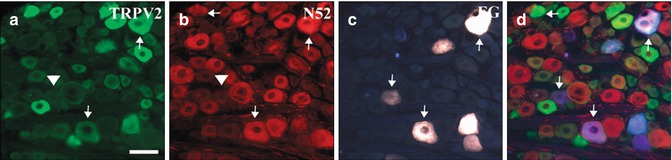

Fig. 6.7
TRPV2 (a) and neurofilament (b) expression in trigeminal ganglion neurons innervating the dental pulp after retrograde labeling with Fluoro-Gold (FG) (c). Tailed arrows point at FG cell bodies that show immunoreactivity for the indicated antigen. Arrowheads highlight cells that contain FG but are immunonegative for the indicated antigen. (d) merged images, arrows indicate FG labeled pulpal neurons. Scale bar = 200 μm. (Used with permission from Gibbs et al. [41])
Perhaps of more relevance to the dental pulp are the cold-responsive channels. Cold allodynia is a common complaint in persons experiencing odontalgia of several etiologies, including pulpitis and dentin hypersensitivity [103, 104]. In fact, an abnormal lingering response to cold is considered the most important diagnostic test for irreversible pulpitis, the clinical diagnosis used when a root canal or extraction is deemed necessary to relieve pain [105]. Two TRP receptors have thus far been identified as molecular sensors for cold, TRPM8, and TRPA1. The TRPM8 receptor is responsive to cool temperatures in the non-noxious range, as well as chemicals that produce a cooling sensation such as menthol and icilin [106, 107]. It has been identified in neurons that innervate dental pulp, both in humans and rodents, although its expression was not correlated with cold sensitivity in humans [108–110]. The TRPA1 receptor is activated by cold temperatures in the noxious spectrum and is also a detector of environmental irritants and pungent compounds such as mustard oil [111, 112]. Like TRPV1, the TRPA1 receptor activity can be modulated by the signaling of several inflammatory mediators including bradykinin [113]. TRPA1 is highly expressed in neurons innervating the dental pulp and may be upregulated in teeth with painful pulpitis [109, 114, 115]. Although both TRPM8 and TRPA1 are interesting novel targets for treating the pain of pulpitis, further work is needed to understand their role in pain transduction within the dental pulp.
6.6 Sodium Channels
Voltage-gated sodium and potassium channels are needed for the generation of action potentials to convey peripheral sensory input into the central nervous system. These channels are termed “voltage gated” as the channels undergo a conformational change in response to application of a voltage, leading to sodium influx and membrane depolarization. There are several subtypes of sodium channels, some of which are expressed in specific subclasses of sensory fibers, including pain fibers, which make them potentially favorable targets for prospective therapeutics [116, 117]. Sodium channels are characterized as being either tetrodotoxin resistant (TTX-R) or tetrodotoxin sensitive (TTX-S), with the TTX-R current mediated by the Nav1.8 and Nav1.9 channels [118, 119]. Studies utilizing mouse genetics to knock out the receptor completely, or to make the neurons expressing the receptor susceptible toxins and subsequent ablation, have shown that the Nav1.8 channel is required for the transmission of painful cold stimuli, mechanical pain, and mechanical and thermal hypersensitivity after inflammation [118, 120, 121]. The channel is also expressed at higher levels under inflammatory conditions, and increased expression of Nav1.8 has been demonstrated in human dental pulp in persons experiencing painful pulpitis [122–125]. Importantly, the channel has also been shown to reduce the efficacy of lidocaine to block nerve transduction. Thus, the upregulation of Nav1.8 within nerves innervating the dental pulp during pulpitic states could contribute to the clinical challenge of achieving adequate local anesthesia during dental procedures.
Another interesting molecular target in the sodium channel family is the TTX-S channel Nav1.7. The importance of this channel to pain was convincingly demonstrated by the identification of genetic mutations of this channel in humans that led to either a gain in function or loss of function of the receptor that was clearly linked to very unique pain symptomatology [126]. Persons with a loss of function mutation were found to demonstrate congenital insensitivity to pain, i.e., they are unable to detect any type of painful stimulus [127]. These patients highlight the importance of pain perception to survival, as they tend to have shortened life spans due to gross injuries sustained because of their inability to detect tissue damage. Moreover, persons found to have a gain in function mutation were found to suffer from chronic ongoing spontaneous pain with an intense burning characteristic. The channel Nav1.7 is found to be upregulated in many animal models of inflammatory pain and also in humans with painful pulpitis [128, 129]. Based on these findings, both the Nav1.8 and Nav1.7 channels are appealing targets for further investigation of the pain mechanisms originating from the dental pulp.
6.7 Autonomic Innervation
The autonomic nerves of the dental pulp belong to the sympathetic division of the autonomic nervous system. Parasympathetic fibers do not seem to innervate the tooth pulp [130]. The sympathetic axons of the dental pulp have their cell bodies in the superior cervical ganglion (SCG). They mainly project to the radicular pulp and form plexa along the blood vessels, while the odontoblast and subodontoblast layers seem to lack a sympathetic innervation [131, 132]. The main sympathetic functional output in the pulp is related to blood vessel constriction. Thus, stimulation of these nerves, or injections of sympathetic transmitters, causes a robust fall in pulpal blood flow [74, 133].
The distribution and density of pulpal sympathetics in mammalian teeth has been estimated with different methods and with varying results. It is conceivable that the extent of sympathetic innervation of the pulp varies between species. Thus, when monoamines have been targeted as markers of sympathetic transmitters using formaldehyde-induced fluorescence, positive nerve fibers were observed in pulps of humans, rabbits, and cats but not rats [132, 134]. Accordingly, the proportion of unmyelinated axons in rat molar pulps was not altered by sympathectomy [35], and retrograde tracer studies demonstrated that very few neurons in the ipsilateral superior cervical ganglion of the rat had projections to the rat molar pulp [135]. Immunohistochemistry has shown that antibodies against neuropeptide Y (NPY), a well-known marker of the sympathetic nervous system, label nerve fibers that line the blood vessels of normal human [75, 131], cat, and rat pulps [74, 131, 132]. Another sympathetic nerve marker, tyrosine hydroxylase (TH), is expressed in both rat [17] and human [39] pulps. Nonetheless, these data should be interpreted with some care, since TH is expressed also in a population of sensory nerves [136]. This is true for NPY as well, which is upregulated in sensory pulpal nerves as a response to challenges such as injury [137] or neuropathy [138]. To conclude, sympathetic stimulation of the dental pulp provides effective vasoconstrictor machinery in mammalian tooth pulps, although the numbers of intrapulpal sympathetic axons involved seem to vary between types of teeth as well as between species. Furthermore, it cannot be excluded that in some cases this mechanism is partly executed through sympathetic fibers on extrapulpal blood vessels, which would escape detection in structural studies of the pulp.
The sympathetic nervous system has an influence on the immune system, through local release of various molecules (see [139]). In sympathectomized rat pulpal tissue, granulocyte recruitment was impaired during experimental orthodontic tooth movement [140]. In line with this, electrical sympathetic nerve stimulation recruited such cells to the pulp. Moreover, immunoglobulin-producing cells were recruited to normal uninflamed dental pulps bilaterally after unilateral sympathectomy. Consequently, pulpal sympathetic nerves appear to play an important role in monitoring and influencing immunocompetent cells in states of infectious/inflammatory challenges to the dental pulp. However, it seems to be unclear as to whether sympathetic activity increases or reduces the severity of different types of inflammation. Thus, resection of the SCG in rats reduced abscess formation after molar pulp damage, but only at short time points. After longer periods, there was no difference in extent or severity of inflammation when compared to controls [141]. Similarly, conflicting results exist with regard to the degree of reparative dentin formation in sympathectomized inflamed teeth [141, 142].
6.8 Generator Mechanisms of Sensory Pulp Nerves
Weak mechanical stimuli such as air puffs and water spray, which are innocuous when applied to, e.g., the skin, evoke intense pain when directed at exposed dentin [143]. It appears unlikely that this is due to direct stimulation of dentinal nerve endings, since these terminate far away in the initial pulp-adjacent segment of the dentin. The hydrodynamic theory holds that force applied at the outermost end of dentinal tubules is transmitted to the sensory transduction apparatus deep inside by mechanical displacement, i.e., flow, of the fluid that the tubules contain [144, 145]. A prerequisite is then that the nerves that are stimulated by these very weak forces are LTMs (provided that they are not sensitized by, e.g., inflammation), since no obvious amplification mechanism is present. This fits well with the data that many if not most dentinal afferents are not classical nociceptors, but rather LTMs. An overwhelming majority is probably A-fibers, but low-threshold C-fibers could theoretically also contribute. The mechanical detection of dentinal fluid movement would require mechanosensory membrane receptors/ion channels in the dental LTM afferents. A number of such molecules have by now been identified in pulpal primary nerve cells. Among these are epithelial sodium channels (ENaCs), ASIC3, TREK1, and TREK2 [114, 146]. Furthermore, members of the TRP family of ion channels, which have been implicated in mechanosensation, are expressed in pulp-innervating trigeminal ganglion neurons, including TRPV2 and TRPA1 (see [147–149]. However, the individual contribution and possible coordinated action of these and perhaps additional membrane sensors remain to be elucidated.
6.9 The Odontoblast as a Putative Pulpal Transducer Cell
The hydrodynamic theory, propagated more than 40 years ago, still provides an attractive model to explain the mechanism behind the sharp and immediate pain that is elicited by various stimuli on dentin. However, it leaves several issues with regard to, e.g., hot and cold sensitivity, in the pulp unresolved. In some cases there seems to be no relationship between pain sensation and movement of dentinal fluid after cold stimulation [150], although some authors claim that distal movement of the fluid in response to cold stimulation is more rapid than proximal movement by hot stimuli, which could affect sensory thresholds [151]. This raises the possibility that additional mechanisms might be activated to convey sensations when teeth are challenged by thermal and perhaps also other stimuli. Very recently, several lines of evidence have pointed to the likelihood that the odontoblast has a role in sensory transduction from teeth, although this is not yet conclusively shown. Thus, calcium imaging studies have demonstrated that human odontoblasts express functional TRPM8, TRPA1, and TRPV1 channels [152]. This indicates that odontoblasts could mediate thermal stress, in concert with sensory nerves in teeth. Furthermore, odontoblasts also express mRNA or protein for mechanosensitive ion channels such as the TREK-1 and KCa potassium channels, which could suggest a mechanosensory function as well [153–155]. An additional electrogenic sensor of stretch activation function of odontoblasts might be accomplished by the recently characterized primary cilia of these cells [156]. Finally, and importantly, odontoblasts express functional voltage-gated sodium channels, which would enable them to become electrically excitable. They also express mRNA for major subunits of ionotropic glutamate receptors (NMDARs), which potentially might be used to generate action potentials [157]. Other sensory cell-related genes present in odontoblasts, again with putative roles in stimulus transduction, include those that code for parvalbumin, the membrane adaptor protein harmonin, the neuronal calcium sensor-1 [6], and synaptic vesicle protein 2b [158].
As seen from this discussion, there is mounting evidence that odontoblasts can respond to sensory stimuli and become electrically excited. However, there is still no reliable proof for the presence of a system, synaptic or other, that translates odontoblast activity into afferent nerve fiber signaling. An interaction that involves ATP is conceivable since purinergic pulp nerve fibers [2, 159] seem to become sensitized by ATP from pulpal cells following inflammation or injury [160, 161]. This may well involve odontoblasts, but is apparently not a sensory cell/nerve-specific mechanism.
6.10 Connectivity of Sensory Tooth Pulp Nerves
The central branches of TG neurons travel via the trigeminal root to the brain stem. Subnucleus caudalis of the spinal trigeminal nucleus is seen as the major nociceptive relay of the trigeminal brain stem complex, since it receives an immense input from pain-transmitting axons that innervate the orofacial region [162, 163]. Morphological investigations using tracing techniques from the tooth have shown that pulpal afferent terminates predominantly in the superficial laminae of subnucleus caudalis, but also in its deep laminae [164–166]. Furthermore, many dental pulp fibers have their central endings more rostrally, especially in the trigeminal subnuclei interpolaris and oralis. When tooth pulps are electrically stimulated, the responses of postsynaptic neurons in all three spinal trigeminal subnuclei correspond to the anatomical findings [162] and largely agree with what would be expected from nociceptors. This is remarkable, since most pulpal sensory afferents have anatomical and electrophysiological characteristics of LTMs and not primary nociceptive neurons. However, since pulpal axons do have the capacity to deliver pain messages to higher brain centers even upon very weak and subtle stimulation, they would have to terminate synaptically on spinal trigeminal nuclei neurons in order to connect into the pain-mediating network.
The fact that pulpal afferents are LTMs whose signals evoke pain rather than touch, due to idiosyncratic connectivity and/or neurotransmitter content, makes them unique among pain-mediating neurons. Since they have very different characteristics from classical nociceptors, we have proposed a novel definition, “algoneurons,” for peripheral neurons that, when activated, evoke a sensation of pain. In contrast to the term nociceptor, the term algoneuron focuses on the sensory effect of the afferent’s signal and not its response properties. According to this, a majority of trigeminal tooth pulp neurons are low-threshold mechanoalgoneurons [38].
In the thalamus, tooth pulp-driven neurons have been identified in ventral posteromedial (VPM) and mediodorsal (MD) nuclei [167]. Considering even higher CNS levels, functional magnetic resonance imaging (fMRI) has demonstrated that painful electrical tooth pulp stimulation leads to bilateral activation of S1, S2, and the insular region of the cerebral cortex. The cingulate gyrus is also activated, as well as motor and frontal areas including the orbital frontal cortex. Tooth pulp pain involves a cortical network, which in several respects appears to be different from that activated by painful stimulation of a hand [5]. Seemingly specific tooth pulp projections to the somatosensory cortex were also shown with magnetic field recording methods. Here, the latencies clearly indicated that the input came from intradental Aβ fibers [168].
6.11 Aging of Pulpal Nerves
With increasing age odontoblasts shrink, apparently due to changes in autophagy [169]. However, secondary dentin formation continues at a slow rate during the life of the tooth, causing a gradual reduction of the pulpal space. This may be aggravated by irregular dentin formed in response to external stimuli. Concomitant with this, a protracted phase of age-related axonal alterations and axon loss occurs. In parallel, there are changes in pulpal nerve cytochemistry. Some of these likely are responses to wear and/or trauma, since they are typically seen proximal to nerve injuries [170, 171]. Pulpal nerve deterioration in senescence is paralleled by a reduced sensitivity to electrical pulp stimulation in human subjects [172].
6.12 Neurotrophins/Receptors in Pulpal Nerve Plasticity
In addition to their important role in establishing innervation of pulpal tissues during development, the neurotrophins and their respective receptors are critical in maintaining the unique phenotype of pulpal afferents in the mature pulp and are important mediators of neuronal plasticity in response to injury. Nerve growth factor (NGF) is the most studied neurotrophin, and indeed all pulpal neurons are at some point dependent on NGF. The receptors for NGF include the high-affinity tyrosine kinase receptor trkA and the low-affinity neurotrophin receptor p75. The importance of the trkA receptor to pulpal innervation is highlighted by the finding that sensory and sympathetic innervation of the dental pulp is eliminated in trkA knockout mice [25]. In the mature pulp, many afferents lose their dependence on NGF with many of the larger fibers becoming dependent on glial-derived neurotrophic factor (GDNF) by expressing the GDNF receptor GFR-α1 [87, 173]. Neurotrophin and neurotrophin receptor expression is altered by the presence of injury and inflammation in the pulp. For example, an upregulation in NGF is observed in pulpal fibroblasts after dentinal injury and is thought to promote sprouting of pulpal afferents [174]. Importantly, neurotrophin expression at the site of injury affects the transcription of genes encoding neurotransmitters, receptors, and ion channels that are key to pain transduction including CGRP, SP, TRPV1, TRPA1, and Nav1.8 [175, 176]. This plasticity is thought to contribute to the hypersensitivity and spontaneous pain that occur after injury [177].
6.13 Neuroplasticity in the Peripheral and Central Nervous System Subsequent to Pulpal Injury
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


