The term pathology can be all encompassing and strictly speaking is defined as structural and functional deviations from normal that constitute disease or characterize a particular disease. In essence, this term can and often does refer to diseases that are aplastic/hypoplastic, neoplastic, traumatic, iatrogenic, or developmental in nature. For the purposes of this chapter, we will limit our discussion to neoplastic and dysplastic diseases.
Although craniofacial surgery developed from the need for comprehensive treatment of congenital pediatric deformities and traumatic injuries, the application of such principles to the treatment of neoplastic disease soon followed. Cranio-maxillofacial (CMF) pathology presents the surgeon with the challenges of gaining access, achieving oncologic margins, and preserving vital structures, along with performing functional and esthetic reconstruction. More specifically, tumors that affect the anterior cranial fossa, the anterior skull base, and the infratemporal fossa are often the domain of the maxillofacial surgeon and require the assistance of a neurosurgeon. Oftentimes, more challenging than the approach to the tumor is the determination of resectability and whether the burden of morbidity outweighs the benefit of complete resection. The role of endoscopically assisted and image-guided resection of tumors is increasing, as is the potential for improved functional and esthetic outcomes.
CRANIOFACIAL GROWTH AND DEVELOPMENT
Cranial and facial growth and development result from complex interrelated and interdependent processes, with no single craniofacial component being self-contained or self-regulated. Growth occurs as a result of a combination of remodeling and displacement. Three primary fields of craniofacial growth and development have been identified: the brain and basicranium, the airway, and the oral region. The basicranium acts as a template to establish the shape and perimeter of facial form. The facial and pharyngeal airway space is determined largely by its surrounding parts. Enlow refers to the airway as the keystone for the face, whose growth is determined in part by both genetics and function, which acts to stabilize the remaining components of the face. The oral region then assumes any basicranial asymmetry or compensates for the asymmetry by its own developmental process. The effect of extirpative surgery or tumor treatment on facial growth and development may be significant although unavoidable if efforts are made to achieve cure. One must consider these factors, not only when planning treatment but also when providing long-term follow-up because subsequent deformity may have a considerable psychosocial impact.
IMAGING
Improvements in the quality of imaging and in our ability to manipulate images, whether it is to plan surgery, to guide the resection of a tumor in real time, or to fabricate a medical model, have had a profound impact on the treatment of CMF pathology. Imaging is particularly helpful because the full extent of the tumor is many times not assessable by physical examination alone. The craniofacial region is relatively dense with vital structures, and appropriate preoperative images help to further elucidate their relationship to the pathologic process. This can be critical for determining the potential to resect the lesion.
Typically, imaging modalities used in the workup of CMF pathology include computed tomography (CT) and magnetic resonance imaging (MRI), with an increasing role for positron emission tomography (PET) and PET-CT. Occasionally, ultrasound, angiography, and bone scans may play a role as well. At minimum, a high-resolution CT or MRI is necessary for appropriate planning. The need for contrast is determined on a case-by-case basis. Software is readily available for the surgeon to use in reformatting images into the standard axial, coronal, and sagittal views, as well as into 3D images for CT scans. In general, CT is less expensive and is preferred for detailing bony pathology and anatomy, tumor bulk, and node involvement. MRI is commonly considered to be superior for assessment of purely soft tissue lesions, for detailed evaluation of nerves and vessels, and for evaluation when the lesion involves the brain or dura.
The use of PET scanning continues to increase rapidly in the field of oncology. It often is most useful in situations where conventional imaging yields equivocal results, as in the previously treated patient. PET scans help to differentiate surgical change from recurrence and reactive nodes from those with metastasis. Overall, PET has been found to be more sensitive and specific than conventional imaging in the evaluation of regional node disease. PET also has value in evaluating patients for unknown primary tumors, synchronous primaries, and distant metastases.
Angiography is used routinely for the evaluation of vascular lesions. Although MR angiography is becoming increasingly sophisticated in the diagnosis of such lesions, conventional angiography offers the benefit of interventional capability should the lesion mandate embolization. Angiography is also useful for determining the extent of carotid involvement of either direct tumor extension or metastatic disease. If carotid resection is deemed necessary, a carotid occlusion study can be performed to assess the patient’s ability to function when he or she is reliant on collateral circulation from the unaffected side via the circle of Willis.
With continuous improvement in imaging techniques, the envelope of surgical possibility becomes increasingly limitless. By integrating surgery with the real-time manipulation and guidance of imaging, we are able to visualize various images simultaneously, to see structures that are otherwise only visible through operative access, and to improve the accuracy of surgery performed in difficult anatomic regions. Potential advantages might include smaller incisions, direct access to specific localized areas, and less morbid or invasive operations.
ANATOMIC CLASSIFICATION
For the planning of craniofacial tumor surgery, Jackson classified the skull base into an anterior region and a posterior region to describe tumor location. The anterior region includes only the anterior cranial fossa. The posterior region was further broken down into anterior, central, and posterior segments relative to the petrous portion of the temporal bone. Others have elaborated further on the site of origin (oral, nasal, cranial, etc) and the direction of extension (craniofacial or faciocranial) and have let this be their guide when approaching CMF tumors. In this chapter, we discuss pathology of the anterior fossa and anterior skull base, the infratemporal fossa, the midface, and the mandible.
Within the facial skeleton, a simple anatomic description modified by degree of extension helps one plan most accurately the surgical extirpation and the required reconstruction.
CMF ACCESS
Many approaches to the facial skeleton have been advocated based on location of the tumor, the necessity of safe resection margins, proximity to vital anatomic structures, and anticipated functional and esthetic outcomes. Access surgery involves a multitude of osteotomies and facial incisions that can be tailored for specific purposes; when combined, these procedures produce acceptable safety for oncologic surgery. The craniofacial skeleton is rich in anatomic complexity. Surgery to the deep face, oropharynx, nasopharynx, and anterior skull base requires thoughtful planning. Although a myriad of approaches to the craniofacial skeleton and anatomic tumor classifications have been described, patients and their pathology should be evaluated on an individual basis. The goal of access surgery is to provide an operative environment in which the extirpative surgeon can safely remove tumor. This basic philosophy accounts for the protection of vital structures and the maintenance of facial esthetics. The value of preoperative planning for access surgery cannot be overemphasized and should entail a comprehensive patient evaluation, including high-resolution 3D imaging and a thorough review of the underlying pathology. Conceptualization of a tumor in space relative to surrounding structures is requisite to the operative phase of surgery.
Access to the superficial facial skeleton is relatively straightforward through a variety of common, well-described approaches. The workhorse incision of craniofacial surgery is the coronal incision. It provides broad access to the orbit and upper midface, and it can be modified or combined with lateral orbital, oral, facial, and nasal incisions to provide near-total access to the anterior or lateral cranial base and the facial skeleton. Disadvantages of the coronal incision include the potential for obvious scarring in patients with male pattern baldness, alopecia, and injury to the frontal branch of the facial nerve, which can be avoided with judicious tissue dissection beneath protective layers of the temporalis fascia and avoidance of monopolar cautery. Noticeable scarring can be addressed and somewhat obviated by the use of the stealth incision, which applies a zigzag incision rather than a straight line, thereby eliminating the obvious part in the hair.
Access to the anterior orbit, including the infraorbital rim and floor, can be attained through cutaneous and conjunctival incisions. Skin incisions provide the benefit of improved access in some situations and may offer the most appropriate means of achieving a biologically sound resection. When benign pathology is managed or a contouring procedure (e.g., fibrous dysplasia) is performed, a transconjuctival approach might lead to a more esthetic outcome. Extension via lateral canthotomy/cantholysis or transcaruncular incision gives one near-total access to the internal bony orbit and the lower half of the orbital rim. The medial orbital wall can be approached through a subciliary lid incision, although the transcaruncular approach affords generous access without a skin incision. Tumors in the paranasal sinuses that invade or abut the medial orbital wall typically require both a skin incision along the lateral nasal wall and a complementary osteotomy. The Weber-Furguson approach, lateral rhinotomy, and medial maxillectomy are commonly employed in this circumstance.
Surgical access to the lower bony midface can be achieved by means of standard vestibular incisions. The midface degloving procedure extends exposure comfortably to the inferior orbital rim and nasal dorsum, and when combined with additional incisions and a craniotomy, this procedure can be used to access the anterior skull base and sinonasal regions without the need for facial incisions. Fundamental knowledge of closed rhinoplasty techniques is applied; these include intercartilaginous incisions that are extended circumferentially within the nares and through the septum to liberate the nasal tip, upper lip, and oral mucosa from the underlying maxilla and nasal structures ( Figure 48-1 ). More generous and direct access to the midface can be achieved through a Weber-Ferguson incision.
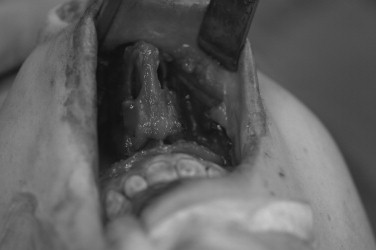
Access to the mandibular body and symphyseal regions can be achieved through mucosal and cervical incisions. Knowledge of the path of the facial nerve in the neck is essential for protecting its marginal mandibular branch. The utility incision for mandibular access is an apron-style incision that is designed in a skin crease at least 2 cm below the inferior border of the mandible. Elevation of the facial vein at the posterior lateral aspect of the submandibular gland, in the manner of Hayes Martin, generally affords protection of the marginal mandibular branch without the need for extensive nerve localization or dissection. Tumor in the mandibular angle, ascending ramus, and pterygomasseteric portion of the mandible requires more in-depth planning, depending on the extent of the tumor and the need for appropriate surgical margins. Access to the condyle for complete hemimandibulectomy generally can be achieved through a cervical incision. The preauricular approach and the coronal approach can be used adjunctively with bulky tumors confined to the head of the condyle. An apron-style incision can be extended to include the lip-split modification and mandibulotomy, which afford direct visualization of the pterygoids and medial aspect of the condyle. Vertical compartment resection of the mandible provides en bloc removal of the vertical ramus and is reserved for tumors that infiltrate the muscle of the pyterygomasseteric sling. It requires a subfacial parotid dissection in the plane of the masseter muscle or a standardized facial nerve dissection; both approaches carry the risk of injury to the buccal branches of the facial nerve.
Small tumors of the oral cavity (less than 4 cm) generally can be treated transorally. Larger tumors of the posterior tongue, tongue base, and retromolar fossa can be approached safely through an apron/lip-split incision and a paramedian mandibulotomy. The osteotomy can be performed in the midline, although lateral placement in the premolar region still preserves the mental nerve and is generally preferable. The inferior border of the mandible is exposed and miniplates are adapted, secured, and then removed. The osteotomy is performed and the indexed hardware is replaced during the reconstructive phase to restore the premorbid occlusion and mandibular continuity. A horizontal ramus osteotomy can be used adjunctively and can sometimes obviate the need for a lip-split incision ( Figure 48-2 ). The stability of rigid fixation is important in that many patients are subjected to external beam radiotherapy before bony healing is complete. A bicortical mini–locking plate provides a significant biomechanical advantage when used at the inferior border. Mucosal incisions should be designed so as not to overlap the osteotomy. A variety of modifications have been suggested to enhance the stability of the reconstructed mandible; however, these are largely unnecessary if AO principles of rigid fixation are applied. Disadvantages of these approaches include added time in the operating room, the potential for non-union in the face of radiation, malocclusion, and trismus. The visor approach entails the raising of bilateral apron-style incisions toward the inferior border of the mandible; these are then connected with sulcular or vestibular incisions. The mental nerves are identified and divided as part of the process. This provides broad access to the lateral tongue and the floor of the mouth. Access to the floor of mouth can be also achieved through a delivery/pull-through approach. Following exposure of the inferior border of the mandible, the geniohyoid, genioglossus, and mylohyoid muscles are divided at their insertions to the mandible. The lingual mucosa then is elevated in a sulcular or vestibular incision on the lingual aspect of the mandible. The entire tongue and floor of the mouth can be safely “delivered” to the neck for tumor removal. The use of a sulcular incision in dentate patients facilitates easy closure.
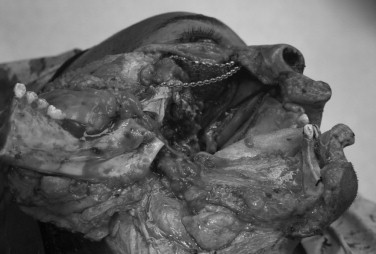
Access to the central skull base and nasopharynx can be obtained through the oral cavity through the use of a standard Le Fort I down-fracture. Lateral osteotomies of the Le Fort may have to be modified to maintain oncologic integrity of the approach, in the form of low osteotomies or limited use of pterygoid separation in the face of gross tumor infiltration. Removal of the posterior wall of the maxilla provides direct access to the nasopharynx. The use of indexed miniplates and or occlusal splints is recommended to restore the occlusion during the reconstructive phase. A two-piece Le Fort I osteotomy broadens access, although the palatal mucosa must be incised to complete the exposure. The relationship of blood supply to the translocated structure(s) should be considered, along with the planned resection, to avoid ischemic complications.
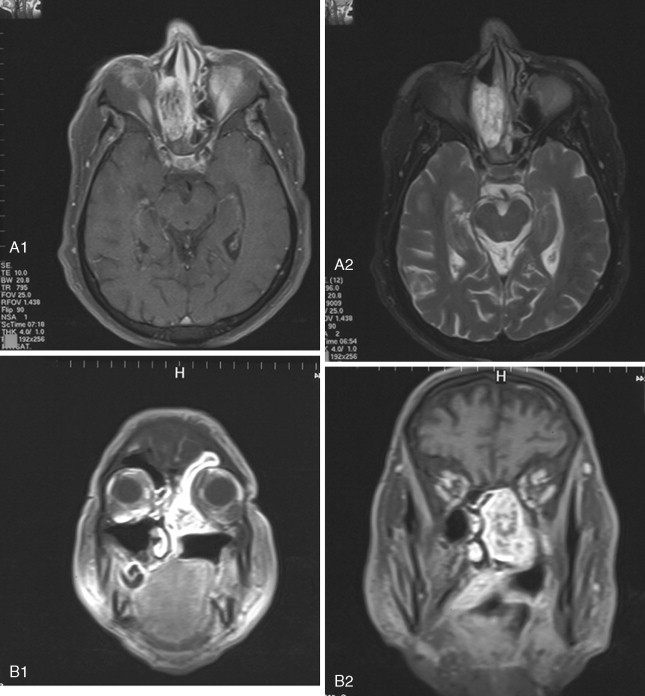
Access to the anterior skull base usually entails a combination of surgical approaches rather than a single approach. Access to the paranasal sinuses and fronto-orbital regions typically involves a facial approach and either a cranial or subcranial approach ( Figure 48-3 ). Each must be tailored to the patient and the location and extension of the tumor. Avoiding a craniotomy has intrinsic advantages, especially in patients with multiple medical comorbidities. The subcranial approach entails the use of custom osteotomies about the anterior craniofacial skeleton. A frontal sinusotomy without removal of the posterior wall allows safe access to the ethmoids and frontal sinus and obviates the need for a craniotomy. Tumors that approach the dura or invade the dura require a formal bifrontal craniotomy. The craniotomy should be planned while both the extirpation and the reconstruction are kept in mind ( Figure 48-4 ). The craniotomy can be extended caudally to include the superior and lateral orbital rims as required for access. The bone flap not only provides access but can be split into calvarial grafts that facilitate bony reconstruction.
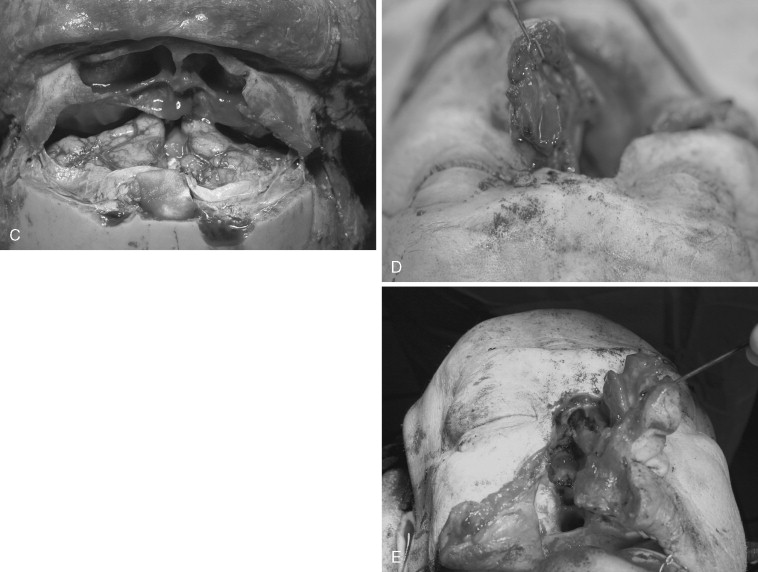
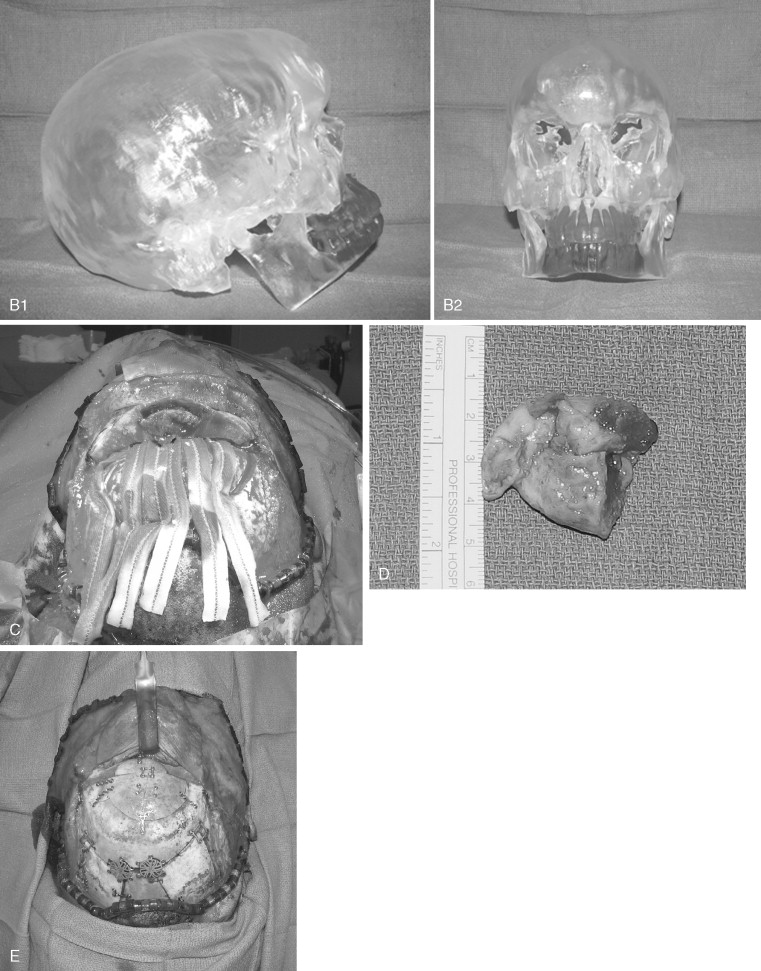
Craniofacial disassembly employs the concept of modular facial anatomic units, developmental embryology, and blood supply. Transfacial swing procedures in which the orbit, maxilla, and mandible are osteotomized in variable combinations allow exposure of the midline of the cranial base. Nasomaxillary translocation involves complete separation of the nasal skeleton and at least half of the maxilla. It is best suited for exposure of the lower clivus. The blood supply of the rotated unit is maintained, and reconstruction is simplified with indexed microplating. Fronto-orbital osteotomies are used for large orbital tumors, internal carotid artery exposure, and orbital apex decompression.
ENDOSCOPIC APPROACHES
Increasingly, endoscopic approaches are being used for tumor resection or to assist in tumor removal, along with conventional approaches. The clear advantage of this technique is the avoidance of facial incisions that might produce permanent disfigurement. In addition, endoscopic approaches may obviate the need for wide periosteal dissections and osteotomies that could interfere with facial growth. In some cases, use of an endoscope allows the surgeon to “look around” the tumor; this often is not possible with conventional skull base access. Rapid healing and return to function and a shortened hospital stay are additional benefits of this technique.
In the hands of a skilled endoscopic surgeon, the potential advantages of the procedure are clear. The learning curve is steep, however, and until one is facile with the technique, possible disadvantages include increased operative time, increased blood loss, decreased access for control of hemorrhage, and incomplete tumor removal. One can always convert to an open procedure when appropriate to maximize the possibility of surgical success.
PATHOLOGY
Benign and malignant tumors involving CMF structures are relatively rare occurrences in children. Treatment of patients with these tumors is based primarily on their biologic behavior, as well as on their location and size. Although cure is the ultimate goal, significant consideration must be given to the functional, developmental, esthetic, and psychosocial consequences of treatment, whether surgical or medical. For surgical disease, immediate reconstruction with grafting and free tissue transfer has become readily accepted.
Benign disease of the jaws generally is classified into odontogenic and non-odontogenic tumors. With the exception of odontomas, odontogenic tumors are rare in children and are classified as epithelial tumors, mesenchymal tumors, or mixed tumors. Of these, the ameloblastoma is the most aggressive.
ODONTOGENIC TUMORS
Odontogenic tumors are thought to arise from the cells that form teeth. They usually are classified as having epithelial, mesenchymal, or mixed origin. Most are benign, and although they can be seen at any age, some occur at a higher incidence in children or behave differently in the pediatric patient; these tumors are discussed in this section.
Ameloblastoma
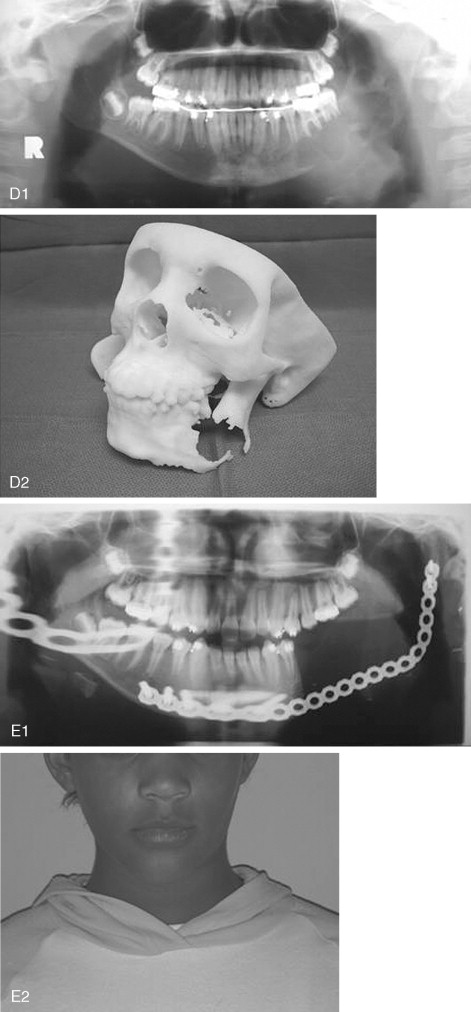
Ameloblastoma is the most common odontogenic jaw tumor, and it is seen most often in the fourth decade. It is benign but aggressive and is divided into unicystic and multicystic/solid types. It is not common in the pediatric population, occurring in only 12-15% of patients <19-20 years, and is very rare in children <10 years. In our original paper, we reported an incidence of 28.9% (11 of 38 cases), but we included in that report adults with recurrent lesions who were originally treated as children; currently (as of January 2007), 21 of our 106 patients are <20 years of age (19.8%). Clinical presentation usually consists of a painless swelling at the angle of the mandible, which may be unilocular or multilocular and may involve buried teeth, mimicking a dentigerous cyst, although root resorption may alert the clinician ( Figure 48-5 ). Usually, buccal and lingual expansion of bone is seen. Frequently, the diagnosis is made as an asymptomatic incidental finding on a panoramic radiograph. However, the importance of recognizing pediatric ameloblastoma as an entity lies in the greatly increased percentage of lesions characterized as unicystic. Unicystic ameloblastomas account for approximately 15% of all ameloblastomas, but they represent 76.5% of pediatric cases reported in Europe and the United States. Unicystic ameloblastoma is a diagnosis that is based on histologic characteristics, not on radiologic appearance. Ackerman et al classified ameloblastomas into four types: Types I and II are intraluminal or within the epithelium only, and types III and IV involve the capsule (mural types). The clinical importance of this is that the solid/multicystic type is aggressive and requires resection to prevent recurrence with soft tissue invasion; low recurrence rates (10-25%) with enucleation alone of unicystic ameloblastoma have classically been described. Most papers group all unicystic ameloblastomas together and do not separate out those unicystic ameloblastomas that exhibit capsular involvement. Whether enucleation alone is sufficient in this group remains controversial. Involvement of the capsule in unicystic ameloblastoma has been reported to occur in 49-81%.
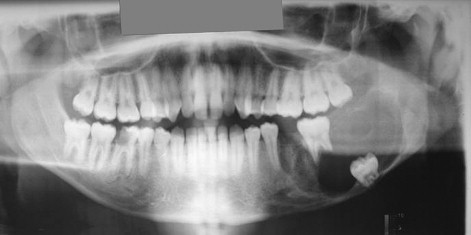
A recent report describes recurrence rates of approximately 80% in unicystic ameloblastomas treated by enucleation, although numbers of study participants were small. A systematic review undertaken to determine which modality of treatment resulted in the lowest recurrence rate yielded rates of 3.6% for resection, 30.5% for enucleation alone, 16% for enucleation and application of Carnoy’s solution, and 18% for marsupialization. The authors concluded that there was only weak evidence to show that resection resulted in the lowest recurrence rate, with the enucleation alone procedure recurring at the highest rate. Marsupialization could not be evaluated. In pediatric cases, we undertake segmental resection with primary microvascular reconstruction for all multicystic/solid ameloblastomas and all unicystic ameloblastomas that are large enough to compromise mandibular integrity or involve soft tissue. Unicystic ameloblastomas with tumor involving just the lining or the lumen of the cyst are treated by enucleation; we advocate resection for mural unicystic ameloblastomas.
Adenomatoid Odontogenic Tumor
Approximately 66% of these tumors occur in patients aged 10 to 19 years. They are common in female individuals and in the anterior maxilla. They present as a swelling in the canine, bicuspid region, and unerupted teeth may be involved. Frequently, these tumors contain small calcifications that may be peripheral; this gives them a mixed radiolucent/radiopaque appearance on x-ray. Patients with these tumors are treated by enucleation, and recurrence is rare. The low recurrence rate of these lesions may be due to the fact that they are hamartomas rather than true tumors, or to their low proliferative rate as seen with a Ki-67 marker. They usually are small and removal is simple, but occasionally, they may present as large lesions ( Figure 48-6 ).
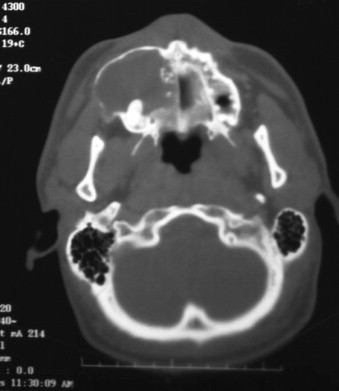
Odontogenic Myxoma
Odontogenic myxoma is most common in patients 20 to 30 years old, but it is seen in children and only rarely in infants. It may appear as a painless swelling of the mandible or maxilla. Radiographically, it may be unilocular or multilocular; however, larger lesions are usually multilocular. Odontogenic myxoma may show root resorption, similar to the ameloblastoma. Often, it contains thin, bony trabeculae, which classically have a stepladder appearance, although it may show an expanded “soap bubble” appearance ( Figure 48-7 ). CT scanning may help to define soft tissue involvement outside the cortical bone. These tumors may infiltrate and may display many of the pathologic characteristics of ameloblastomas; although some authorities have recommended enucleation/curettage for smaller lesions, this procedure leads to recurrence in 25% of cases, and resection is recommended. The authors believe in resection with 1 cm margins, especially for posterior maxillary lesions that can lead to invasion of recurrent tumors into the pterygoids, orbit, and skull base if curetted. Some authors have advocated maxillectomy with narrow margins in cases of pediatric maxillary myxoma.
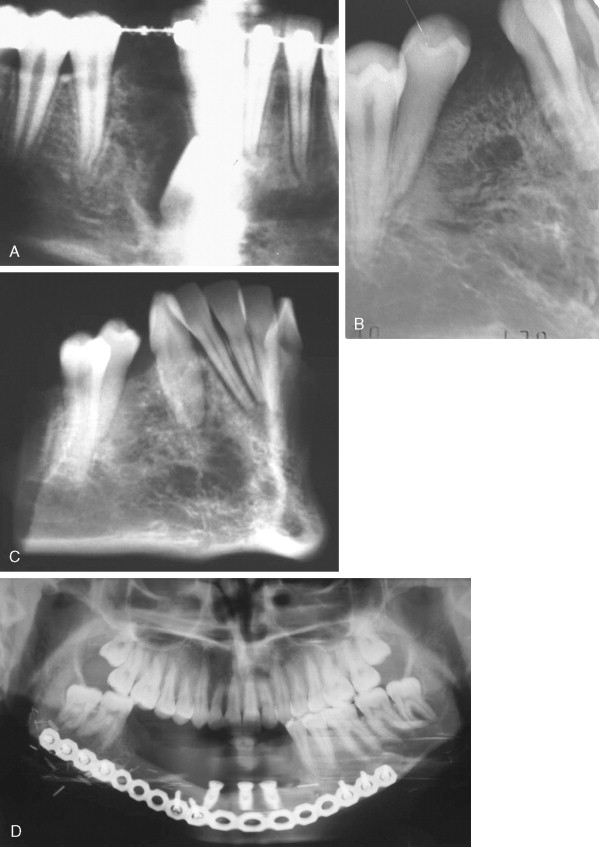
Ameloblastic Fibroma
This tumor contains neoplastic elements derived from both epithelium and mesenchyme. Some of these lesions may be early developing odontomas as opposed to true tumors. They are most often seen in children <20 years of age, and most occur in the posterior mandible either as a swelling in larger cases or as an incidental radiographic finding of a well-defined unilocular or multilocular lesion ( Figure 48-8 ). Many reports suggest that recurrence following simple curettage is rare; however, recent reports have revealed recurrence rates of up to 20% and have recommended complete surgical excision. Although the malignant counterpart, ameloblastic fibrosarcoma, is rare, approximately 50% of cases arise as recurrence of a previous benign ameloblastic fibroma. Although the ameloblastic fibroma usually is treated by curettage or simple excision, recurrent lesions may require resection. In cases of malignant ameloblastic fibroma, radical resection offers the best chance of local control.
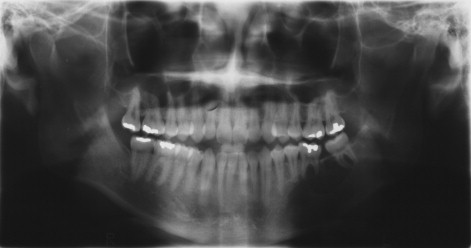
Ameloblastic Fibro-odontoma
This lesion is found in young children, often <10 years of age. It resembles the ameloblastic fibroma histologically but contains enamel and dentin. Although this lesion again may represent a stage of odontoma formation, some lesions continue to grow and behave like a true neoplasm ( Figure 48-9 ). Clinically, they occur as swellings of the maxilla or mandible. On radiography, they are radiolucent lesions that contain differing amounts of calcification and usually an unerupted tooth. Treatment is given by curettage, and recurrence does not occur.
Odontoma
Odontoma is the most commonly seen odontogenic tumor; these lesions are hamartomas that are subdivided into complex odontomas, which are masses of calcified tissue (dentin and enamel), and compound odontomas, which consist of multiple tooth-like structures. These lesions are seen most often in children and frequently are diagnosed as they interfere with normal tooth eruption. Radiologic features may include a few small, tooth-like structures that overlie an unerupted tooth or a large area of calcification with a radiolucent rim and sclerotic border, depending on the type and size of the lesion ( Figure 48-10 ). Simple excision is curative but may be challenging in large cases.
NON-ODONTOGENIC TUMORS
Juvenile Aggressive Fibromatosis
Fibromatoses arise from the superficial or deep musculoaponeuroses of regions of the body, most commonly the abdominal wall (desmoid tumor). However, they may arise in the head and neck, particularly in children <10 years of age, and tend to aggressively infiltrate this location. Gold describes three histologic types: desmoplastic, active, and sarcomatoid. These may be located in bony, paragnathic, or soft tissues. Although these tumors destroy and infiltrate bone, marked proliferation of cortical bone often is seen in paragnathic cases in young patients ( Figure 48-11 ). Perhaps this is due to stimulation of the active periosteum in children. Although some of these lesions are very indolent and slow growing, others infiltrate widely and can grow rapidly, behaving as low-grade sarcomas ( Figure 48-12 ). Because of their poorly circumscribed margins, which are difficult to define clinically or on imaging studies, obtaining good surgical margins is frequently difficult, and recurrence rates of 70% are reported. In purely oral lesions, excluding neck lesions with brachial plexus involvement, recurrence rates of 23.8% have been achieved. It is interesting to note that many authors have commented on the fact that complete excision with negative margins is not always essential for obtaining local control and long-term stability; regression has been reported in incomplete excision. Sacrifice of essential nerves may therefore not be essential. At some head and neck sites, such as the skull base, it may be impossible to obtain clear margins with this disease. In unresectable cases with continuing growth, radiation therapy, chemotherapy, and hormonal therapy all have been attempted with varying success rates. No good prospective trials have identified the best management approach other than surgery for aggressive fibromatosis; these lesions remain a multidisciplinary challenge.
Central Giant Cell Lesions
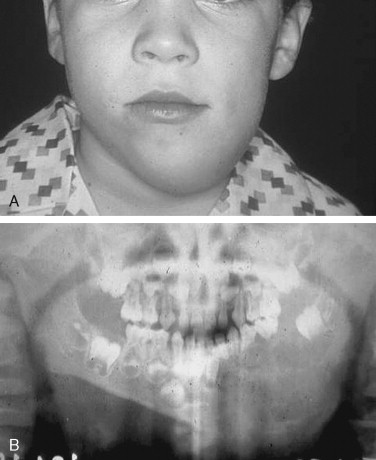
The central giant cell granuloma is an osteolytic lesion of the jaws, which is locally destructive and variably aggressive ( Figure 48-13 ). The mean age of diagnosis is 20 years, and the condition is more common in females (3 : 2). The mandible is involved twice as often as the maxilla, and lesions range from asymptomatic swellings or incidental findings to painful growths with associated paresthesias. Chuong et al, in 1986, classified giant cell lesions of the jaws as aggressive or non-aggressive on the basis of several criteria. Aggressive giant cell lesions were defined as measuring >5 cm, showing rapid growth, and causing tooth root resorption or tooth displacement, cortical bone thinning or perforation, or recurrence after simple curettage. Findings that included a tumor measuring >5 cm or a tumor that recurred after curettage were considered aggressive tumors based solely on these features; otherwise, three of the other signs have to be present for a tumor to be classified as aggressive.
Less aggressive lesions in the jaws are often amenable to simple curettage. Aggressive lesions have reported recurrence rates as high as 70% after enucleation or curettage. Multiple modalities have been used to treat giant cell lesions, including interferon, corticosteroids, or calcitonin, on the basis of the assumption that the lesions are proliferative vascular lesions, that they are inflammatory, or that the osteoclast is the predominant cell type. Results of these treatments when used alone have been mixed. For now, the gold standard of therapy for aggressive lesions is en bloc resection, although Kaban has published promising results on the combination of curettage of the aggressive variant with adjuvant interferon therapy.
Aneurysmal Bone Cyst
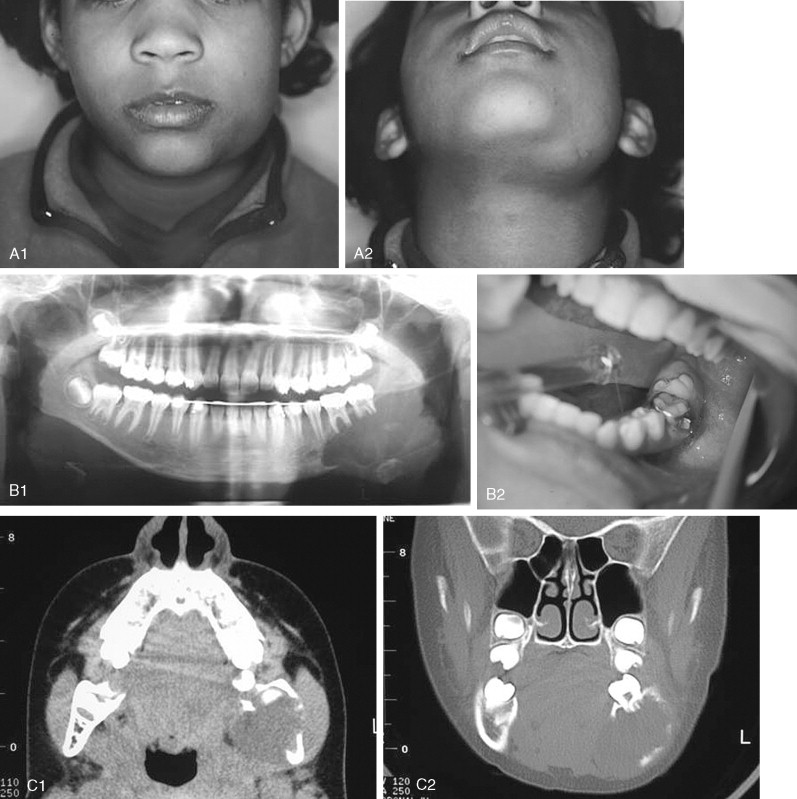
The aneurysmal bone cyst (ABC) is a pseudocyst of bone filled with blood, which can occur as a primary lesion or secondarily as a component of another lesion such as fibrous dysplasia. These “cysts” can be unilocular or multilocular, and they can cause significant expansion of the affected jaw or bone. ABCs are more common in the mandible, but they occur in the maxilla and have been reported in other bones of the face, such as the zygoma, the clivus, and the cranial bones. They may occur in any age group, but they usually are seen in patients <20 years of age. Frequently, a history of trauma precedes development of the lesion.
Aneurysmal bone cysts can be treated by curettage, but they have a high incidence of local recurrence. ABCs often are multilocular and are divided by multiple bony septa. This situation can interfere with curettage, and it explains the many treatment modalities used in their management, which include no treatment, curettage, cryotherapy, excision, and resection. Marginal or en bloc resection should be curative but requires future reconstruction ( Figure 48-14 ).
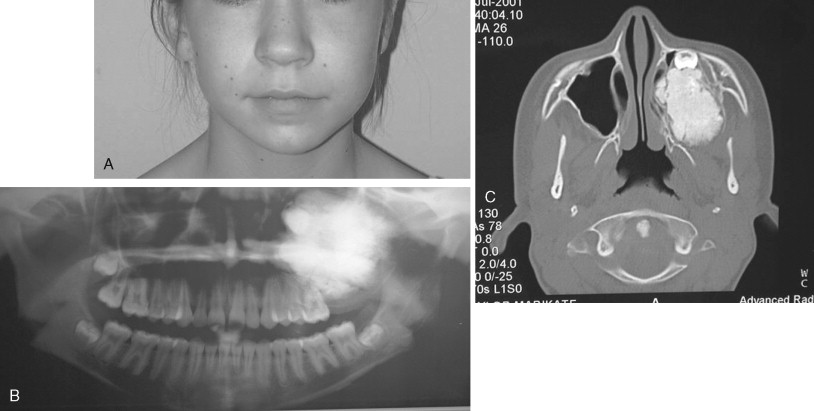
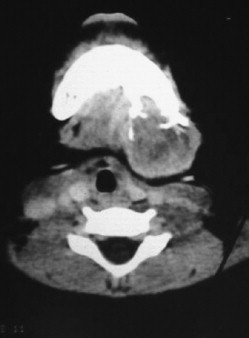
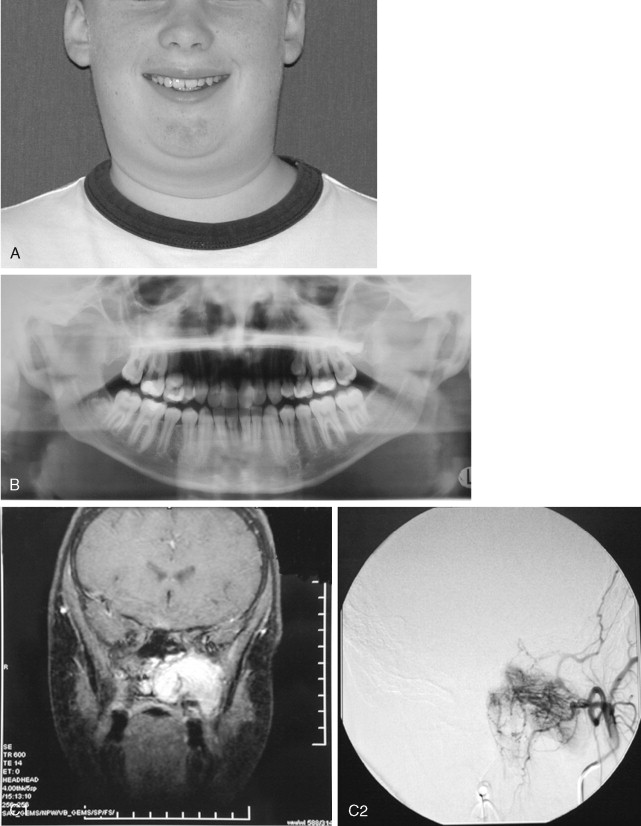
Juvenile Nasopharyngeal Angiofibroma
Juvenile nasopharyngeal angiofibromas (JNAs) are highly vascular benign aggressive tumors that occur in the region of the sphenopalatine foramen of adolescent male patients and account for 0.05% of head and neck tumors. These lesions are non-encapsulated and frequently extend beyond their site of origin to involve the nasal cavity, sphenopalatine fossa, orbit, sphenoid sinus, and middle cranial fossa. The typical presentation is that of painless unilateral nasal obstruction and epistaxis. Intracranial extension and bleeding may result in life-threatening complications.
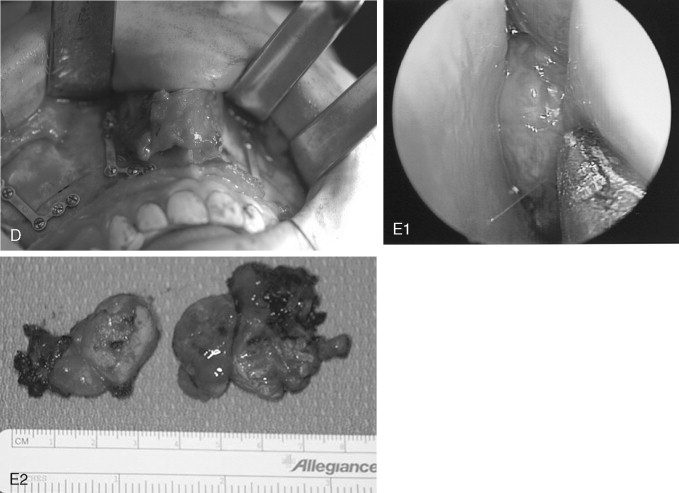
Cure is achieved by complete surgical extirpation, and the approach to the tumor is tailored to the specific anatomic location. Many approaches have been used and advocated, including various facial incisions and skull base approaches. Recently, endoscopic approaches have gained favor as the procedure of choice when feasible. Carrau et al have had good success with endoscopic and endoscopically assisted approaches. Their recurrence rate compares favorably with those historically reported in the literature (13% vs. >20%), and associated complications are few. Regardless of the surgical approach chosen, most centers advocate preoperative angiography and embolization of the major feeding vessels to improve hemostasis at the time of removal. The arterial sheath then can be left in place for assistance in managing difficult to control intraoperative bleeding.
Although the JNA is often within the realm of the ear, nose, and throat (ENT) surgeon, the maxillofacial surgeon frequently contributes to the management of this pathology. Even though it is becoming more common for these tumors to be removed endoscopically, access osteotomies still have a role in the treatment of these benign but aggressive lesions ( Figure 48-15 ).
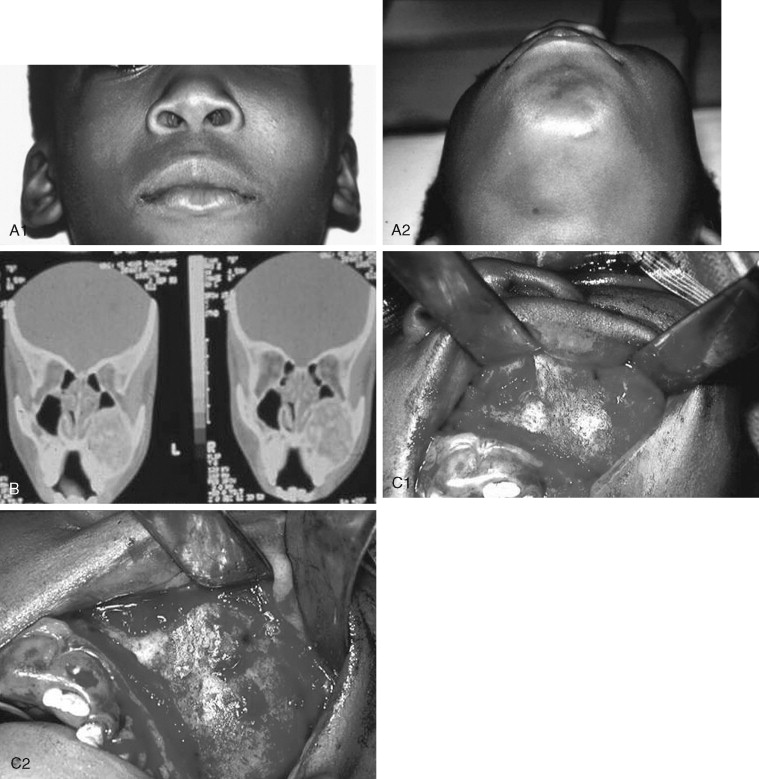
Juvenile Ossifying Fibroma
Juvenile ossifying fibroma (JOF) is a fibro-osseous lesion that is histologically similar to ossifying fibroma and fibrous dysplasia. The lesions are more common in the maxilla than in the mandible, and they are most frequently encountered in the first and second decades of life (age <15 years). It is a variant of the ossifying fibroma and is characterized by rapid progression during childhood. JOF is alternately known as the aggressive ossifying fibroma, and it is distinguished histologically from the more common ossifying fibromas by its cellular fibrous stroma with a network of osteoid rimmed by active osteoblasts. Growth is rapid, occurring over weeks rather than months to years (as seen in the non-aggressive form), and it may cause erosion of the adjacent bone and significant facial deformity and functional impairment. Surgical resection has been advocated for these lesions because the recurrence rate is 30 to 56%. Because of their rapid enlargement and oftentimes large size, facial incisions to improve access may be unavoidable for ensuring complete tumor removal ( Figure 48-16 ).
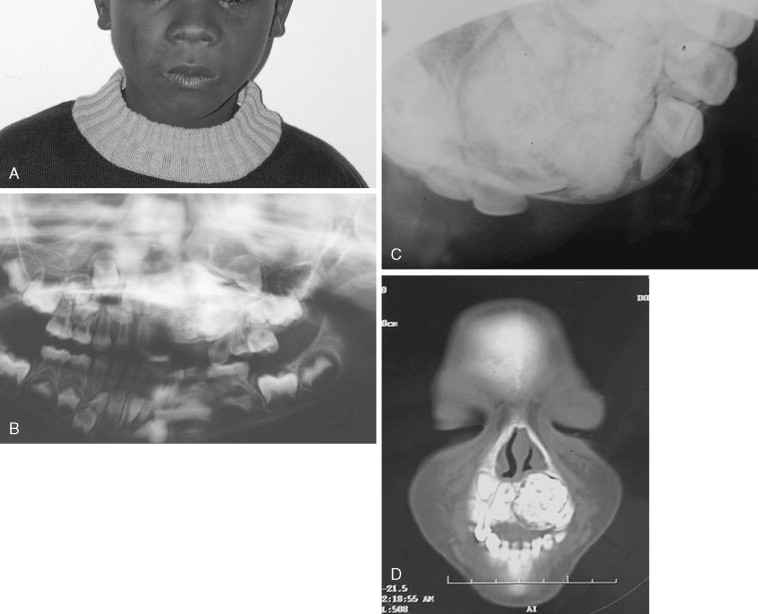
Craniofacial Fibrous Dysplasia
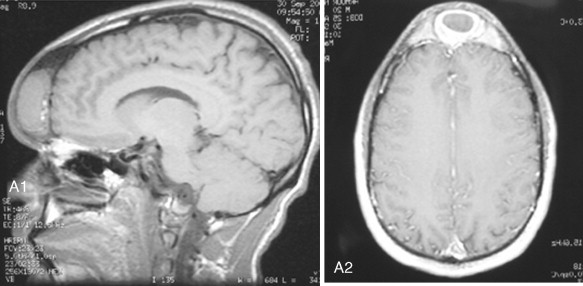
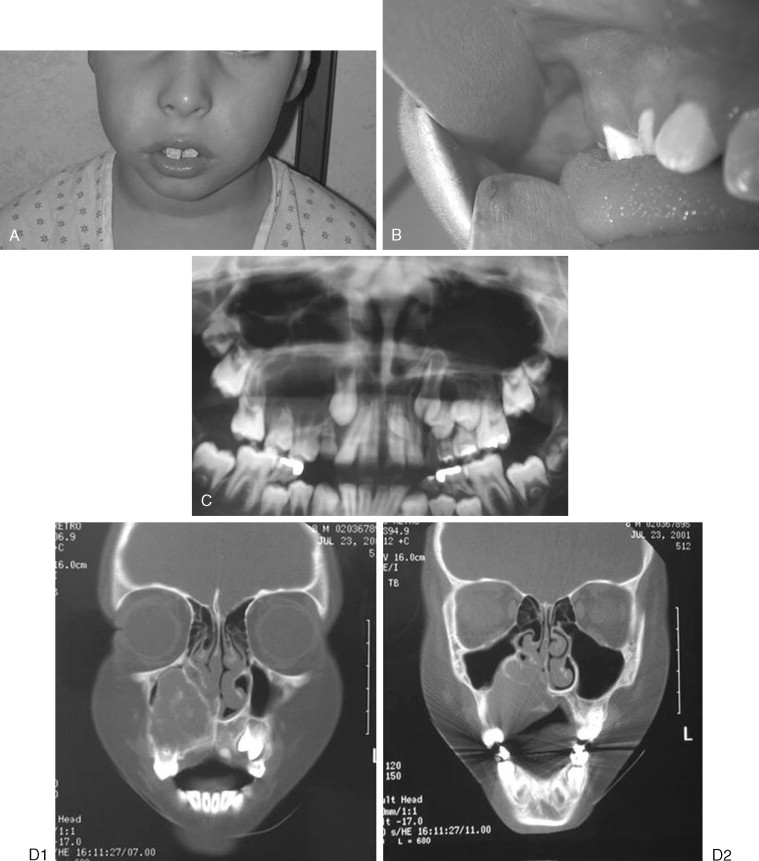
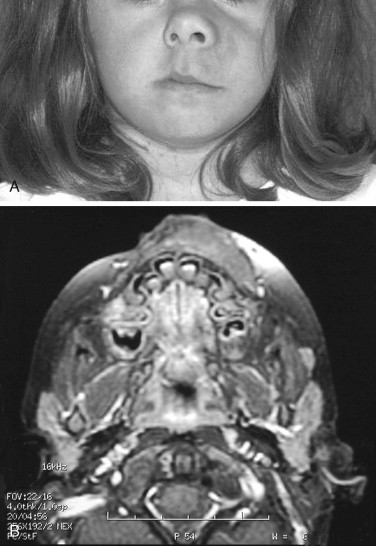
Fibrous dysplasia is a disease in which normal bone is replaced by abnormal but benign fibro-osseous tissue. It was initially described by von Recklinghausen in 1891 and was further characterized by Jaffe and Lichtenstein. This disorder results from a mutation of the GNAS1 gene, which leads to increased intracellular cyclic adenosine phosphate. It can occur as an isolated condition or as a component of McCune-Albright syndrome. The syndrome includes the findings of fibrous dysplasia and café au lait skin pigmentation, in addition to multiple endocrinopathies ( Figure 48-17 ). Fibrous dysplasia can affect single (monostotic) or multiple (polyostotic) bones of the skeleton and commonly involves craniofacial structures. The disease manifests as slowly progressive and painless swellings of bone that result in functional problems related to the area of growth. Although it is largely a benign disease, a low rate of malignant sarcomatous transformation has been reported ( Figure 48-18 ).
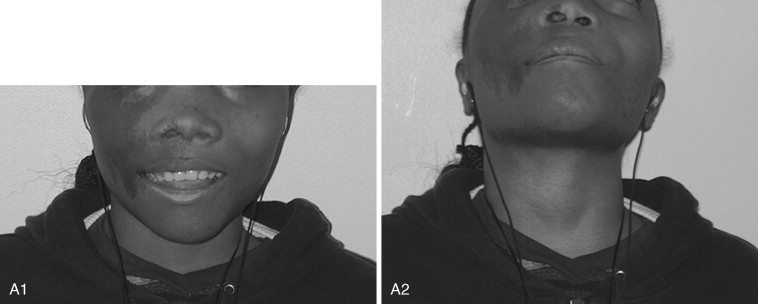
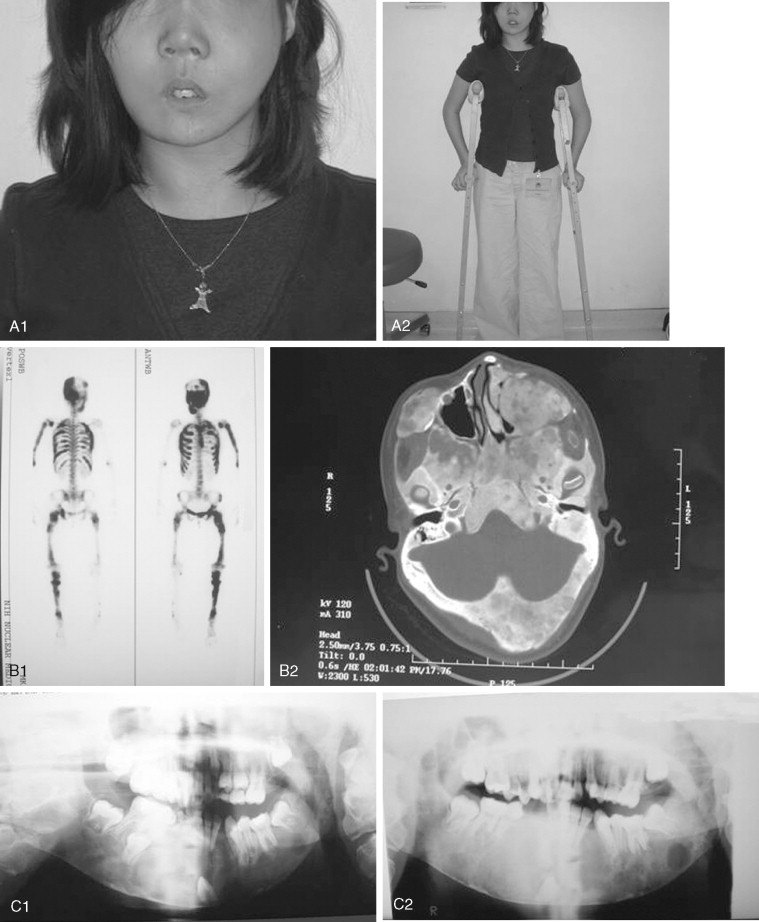
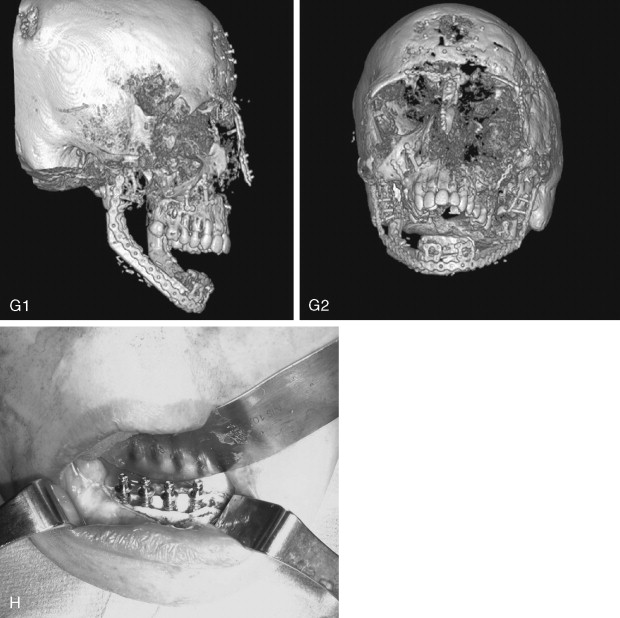
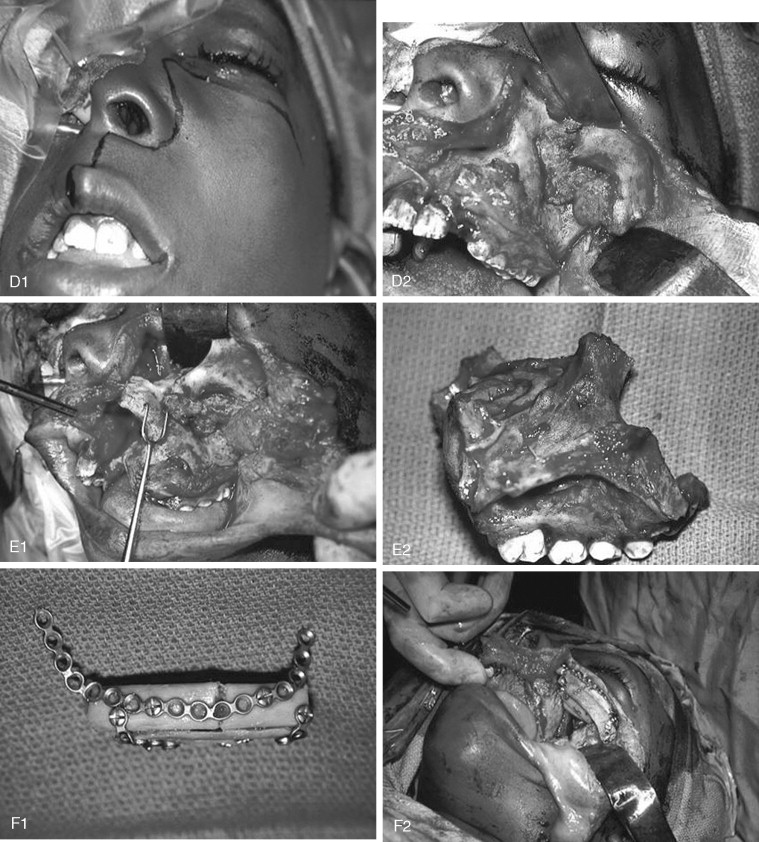
Craniofacial fibrous dysplasia (CFD) can result in severe deformity and significant functional impairment. Complications are generally the result of both the mass effect and the composition of the lesion. Ocular complications include diplopia, proptosis, blindness, epiphora, and exposure keratitis. Loss of color vision often signifies impending visual loss, one of the more devastating consequences of the disease. At one time, prophylactic optic nerve decompression was advocated on the basis of the appearance of CT scans ; however, Lee et al recently recommended close follow-up with symptomatic intervention only, citing a poor correlation between blindness and optic canal narrowing as viewed on diagnostic imaging. Oral manifestations may include malocclusion, loose teeth, limited opening, and impaired mastication. Nasal obstruction and obliteration of the sinuses are also common. Pares thesia and anesthesia can be the result of direct nerve compression; central nervous system effects are also documented when the neurocranium is involved.
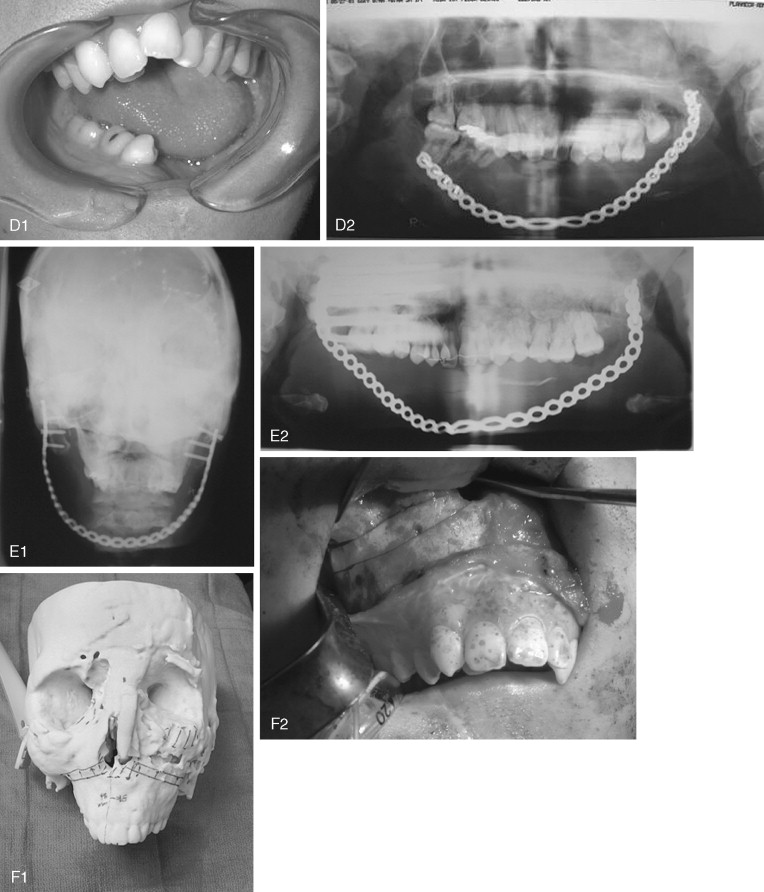
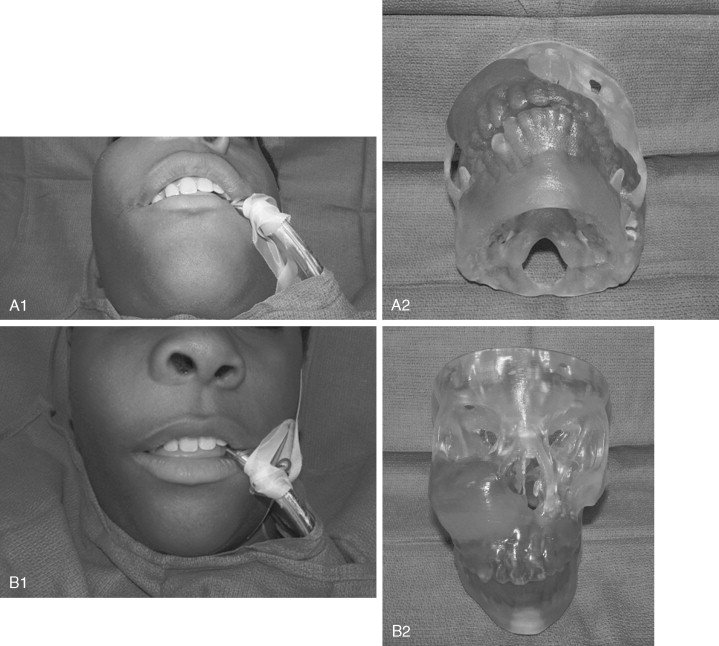
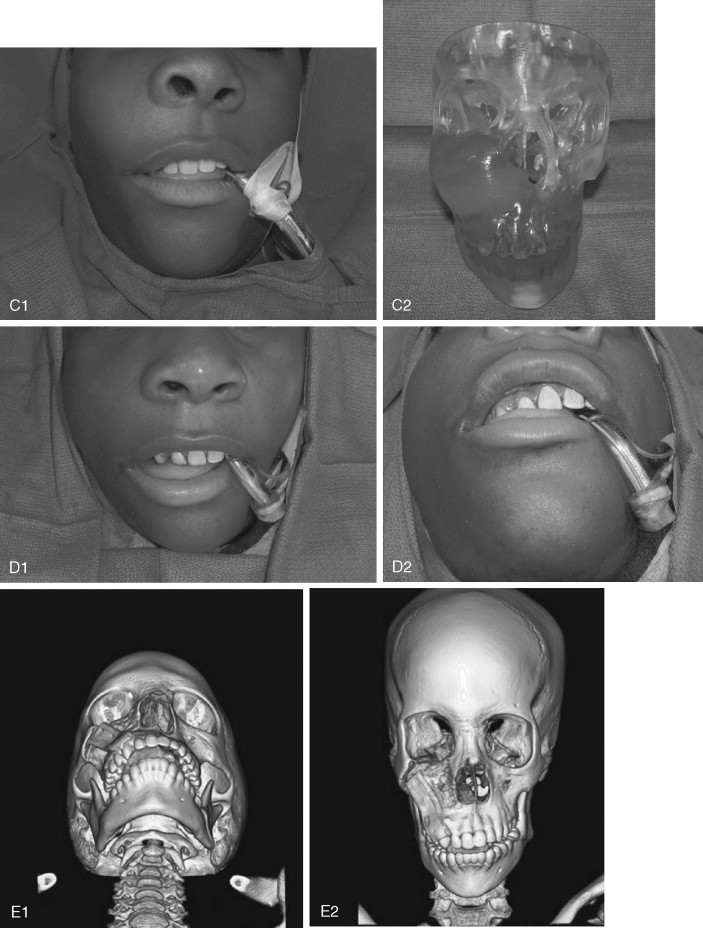
The appearance of polyostotic CFD has been described as that of leonine facies. This condition results from broadening and lengthening of the face caused by dysplastic bone growth. Dysplastic tissues do not grow symmetrically, further worsening the esthetic appearance. The effect, however, can be much more localized, affecting one or several bones of the face unilaterally and resulting in obvious asymmetric swelling. Esthetic concerns can have a significant psychosocial impact in this patient population because the disease frequently manifests during the first and second decades. Because this is a benign condition with low malignant potential, and because the affected areas are so diffusely involved, repositioning osteotomies and bony contouring are the mainstay of surgical therapy ( Figure 48-19 ). Others have described resection and reconstruction with bone grafts, but the authors rarely find an indication for this approach. In general, contouring procedures offer some esthetic and functional benefit without eradicating the disease. The author’s preference is to perform contouring procedures definitively once growth has ceased; however, psychosocial and functional concerns often mandate earlier intervention, with the understanding that revision may be necessary as growth of the lesion continues.
Many groups have documented the value of bisphosphonate therapy in small cohorts of patients with fibrous dysplasia and McCune-Albright syndrome. These studies have shown the beneficial effects of long-term bisphosponate treatment on dysplastic lesions, leading to reduced fracture rate and bone pain and radiologic evidence of long bone lesion healing. Additional randomized controlled trials are needed, however, before the efficacy of bisphosphonates can be advocated.
Depending on the quality of the dysplastic tissue, contouring of facial bones can be accomplished with rotary instrumentation, saw blades, and a variety of chisels and gouges. Osteotomies can be accomplished in the usual fashion and fixated with plates and screws. Sometimes, the soft fibrous nature of the bone can make fixation a challenge. It is important to note that these lesions can be well vascularized and sometimes result in significant blood loss, so cross-matching of blood is indicated for longer procedures. Dental complications, including malocclusion and failed orthodontic therapy, have recently been described. Because the application of orthodontics is often a necessary and useful adjunct to orthognathic procedures, definitive jaw surgery can be all the more challenging in this patient group. With sometimes excessive growth of the facial bones comes slow expansion of the soft tissue envelope. This may take time to redrape following surgery, and despite aggressive contouring, it can produce the appearance of residual deformity.
Neurofibromatosis
Neurofibromatosis (NF) is an autosomal dominant disorder that occurs as one of two genetic variants—NF1 (von Recklinghausen’s disease) or NF2 (central neurofibromatosis). NF1 occurs in approximately 1 in 4,000 live births and often results from a spontaneous mutation. Specific criteria have been determined that result in a diagnosis of NF1. These include two or more of the following signs: café-au-lait spots, multiple neurofibromas (or one of the plexiform type), axillary/groin freckling, optic glioma, Lisch nodules, dysplasia of the sphenoid or a long bone, and a first-degree relative with NF-1 ( Figure 48-20 ). Centrally, tumors of the optic tract are seen in up to 20% of patients, and the most common peripheral tumor is the plexiform neurofibroma.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


