Fig. 3.1
(a) Superior antihelix with guide. (b) Inferior antihelix with guide. (c) Hader bar. (d) Auricular prosthesis
Soft tissue around implants can become inflamed following implant placement, and in many cases, highly polished porcelain is placed on the abutments to aid in maintaining the health of the soft tissue in this area. Patients with auricular defects require implants to assist in retaining the prosthesis. Cyclical loading is not seen in these types of restorations. Hader clips on a bar splint the implants together and allow for adequate stress distribution (Fig. 3.1c, d). An acrylic substructure links the clips to the silicone prosthetic ear.
3.2.2 Orbital Implants
Orbital implants present with complex restorative options given the anatomy. The superior orbital rim is typically a good recipient and can often accommodate implants. The medial aspect of the superior orbital rim often contains the frontal sinus and will not allow for implant placement in this area (Fig. 3.2a, b). In the event of loss of vision in one eye, the depth perception is less optimal, and hence, using magnetic retention of the prosthesis allows patients to place the prosthesis more easily. In addition, the use of magnets allows for less axial force to be placed on the implants (Fig. 3.2c, d).
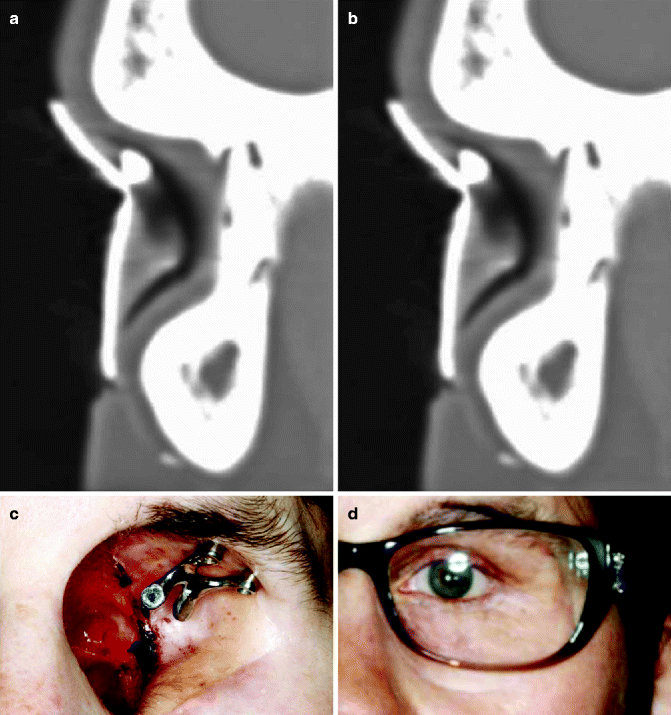

Fig. 3.2
(a) Medial orbit with guide. (b) Lateral orbit with guide. (c) Lateral orbit with magnet attachment. (d) Orbital prosthesis
3.2.3 Nasal Implants
In nasal prosthetic reconstruction, dental implants may be placed in the floor of the nasal cavity. Placing implants in the glabella presents difficulty given the implant angulation and its proximity to the frontal sinus. Instead, the maxilla often has great vascularity and allows for standard maxillary osseointegration. Particular attention must be employed to avoid trauma to the apices of the natural teeth if they are present (Fig. 3.3a). Usually implants must be placed in the premaxillary segment at about a 45° angle due to the long axis of the adjacent teeth. The typical substructure consists of a bar with Hader clips (Fig. 3.3b). These implants are restored with an acrylic substructure linking to the implant bar (Fig. 3.3c).
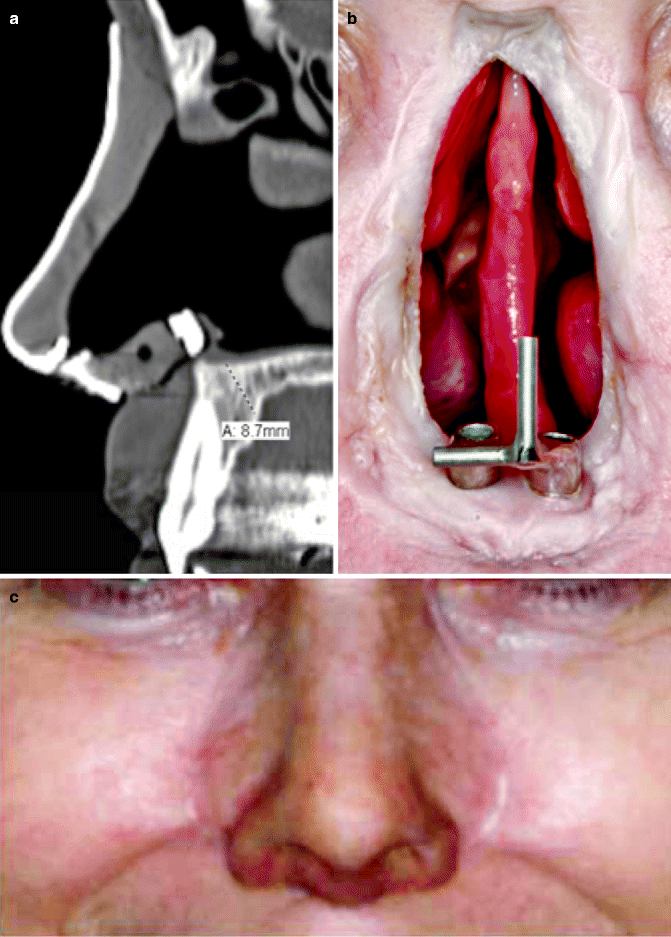

Fig. 3.3
(a) Guide over central incisor. (b) Hader bar. (c) Restored nose
3.3 The Long-Term Stability of Craniofacial Implants
3.3.1 Poor Implant Survival in Orbital Bone
In a retrospective review of 207 craniofacial implants placed in 72 patients, the overall survival rate for uncovered craniofacial implants was 80 %, which was significantly lower than that of the conventional dental implant. The detailed evaluation revealed that the cumulative 6-year survival rate for auricular, piriform/nasal, and orbital implants varied and was found to be 92, 87, and 59 %, respectively (Roumanas et al. 2002). Another study reported similar findings in their craniofacial prostheses patient population, i.e., that the survival of total implants in auricular, nasal, and orbital in congenital, trauma, and oncologic patients was 94.1, 88.9, and 80.8 %, respectively (Visser et al. 2008). Furthermore, in a study involving 153 implants placed to retain 44 orbital prostheses and followed for a mean period of 52.6 months, the overall integration survival rate was 73.2 % (Toljanic et al. 2005). From these studies, while craniofacial implants may offer significant benefits to patients, orbital bone presents a disadvantageous site for implant treatment, and the long-term stability of orbital implants can be unpredictable. No obvious causes to explain the decreased orbital implant survival were found among factors such as radiation, hyperbaric oxygen therapy, or implant location within the orbital bone (Toljanic et al. 2005).
3.3.2 Effect of Radiation Therapy (XRT) on Craniofacial Implants
In a study of craniofacial prostheses retained by implants and adhesive in 10 patients, 5 patients had prostheses retained with implants utilizing a total of 14 implants, and 2 had failed that were placed in irradiated areas (Pekkan et al. 2011). In another study, implants placed in nonirradiated tissues and irradiated tissues were evaluated. In nonirradiated tissues, survival rates for auricular, piriform, and orbital were 94, 80, and 70 %, respectively, with overall survival rate of 85 %. Survival rates in irradiated tissues for auricular, piriform, and orbital were 100, 83, and 27 %, respectively, with overall survival rate of 52 %. Limited numbers of orbital implants in patients were noted (Roumanas et al. 2002). Others reported in nonirradiated fields of the ear, nose, and orbit as 96, 87.5, and 94.1 %, respectively, with overall survival rate of 95.2 %. Implants in irradiated bone were reported as placement pre-irradiation and postirradiation. Survival of implant placement postirradiation of auricular, nasal, and orbital bone was 100, 100, and 28.6 %, respectively. Implant placement survival with pre-irradiation of the auricular, nasal, and orbital bone was 81.8, 83.3, and 79.3 %, respectively (Visser et al. 2008).
These studies indicated that reduction of implant survival in irradiated tissues was rather limited to orbital bone (Fig. 3.4). In particular, orbital implants placed after XRT showed a significantly decreased survival, whereas the orbital implant placed before the radiation therapy sustained their survival albeit at a lower rate than those placed in other craniofacial bones. Case reports describing the failure of orbital bone implants after XRT began to emerge in the 1990s. An increased loss of implants with time after irradiation was noted (Granström et al. 1993).
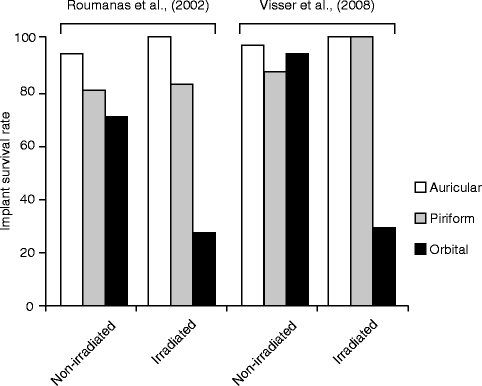

Fig. 3.4
Craniofacial implant survival rate of auricular, piriform, and orbital prostheses reported by two independent studies
3.3.3 Why Do Orbital Implants Fail?
To date, the cause of the selective reduction in the survival of orbital implants, particularly after XRT, has not been elucidated.
The human orbit is composed of the frontal, maxilla, zygomatic, and lacrimal bones. The orbital plate of the frontal bone occupies the superior margin where orbital implants are placed. This bone is a dense cortical bone. This location is also adjacent to the frontal sinus, which limits the implant length and direction.
Craniofacial implants are shorter then dental implants; typically these implants are about 3–4 mm in length. Craniofacial implants are also different from intraoral implants in that the implant surfaces of currently available craniofacial implants are untreated. Many of these implants have collars on the neck of the implant to prevent overseating during placement. Too deep placement would create damage to the underlying dura mater. The short length may partially explain why survival rates are less than that of intraoral dental implants.
Furthermore, the biomechanics of facial prostheses are different from the cyclic loading that intraoral implants undergo. However, implants placed in orbital bone are often subjected to off axial loading with loads applied perpendicular to the implants. These mechanical and anatomical issues may contribute to the loss of orbital implants.
3.3.4 Osseointegration of Craniofacial Implants
Anatomically, craniofacial bones are composed of relatively thick cortical bone and undeveloped trabecular bone. The initial implant stability may be established by the mechanical interaction between implant threads and cortical bone. It is believed that craniofacial implants may largely depend on this mechanical fixation. The orbital implants placed in the thin cortical bone may lack this initial fixation.
Bone remodeling is believed to be higher in trabecular bone than cortical bone, and thus, the biological establishment of osseointegration should be active with trabecular bone. In a preclinical animal study, implants were placed in the frontal calvarial region of nine adult pigs, and it was found that the bone-implant contact reached approximately 31 % at 2 weeks, 39 % after 4 weeks, and 51 % at 8 weeks (Petrovic et al. 2007). Implants placed in craniofacial bones should achieve osseointegration. However, the small trabecular bone compartment in craniofacial bones may be less adaptive for biological implant fixation. It is conceivable that radiation therapy reduces the viability of cells in the trabecular space. This may further impair the establishment of biological osseointegration involving bone remodeling.
It is tempting to speculate that osseointegration of craniofacial implants may highly depend on the mechanical fixation with cortical bone, while the de novo osseointegration in the trabecular space may not be effectively established. Although the rate of cortical bone remodeling may be slow, once the bone resorption is initiated, the loss of mechanical fixation may occur.
3.4 Survival of Intraoral Maxillofacial Implants
Dental implants have been used to retain maxillofacial prostheses such as maxillary obturators. Depending on the configuration of implant placement, size of defect, number of implants, size of implants, and interocclusal space, splinted substructures or attachments are recommended. Splinting implants may allow for more even strain distribution on loading (Yilmaz et al. 2011).
It is important to note that these types of maxillofacial implants are placed in alveolar bone of the maxilla and mandible when there is ample bone. A relatively poor overall survival rate of implants was found at 69.2 %, with 63.6 % for the irradiated group and 82.6 % for the nonirradiated group (Roumanas et al. 1997). The majority of implant failures occurred in the maxilla. This may be partly due to unfavorable implant loading, e.g., a cantilever mechanical loading from a maxillary obturator, which may have a further disadvantageous effect. The maxillary implant survival was further decreased by XRT.
In another study, 446 implants were placed in the maxilla or mandible of cancer patients. In this study, they found that if implants are to fail in irradiated tissues, they will typically fail within the first 6 months. In this study, they found implants had an 84 % survival if the tissues received less than 50 Gy and 71 % if irradiated with greater than 50 Gy. More failures occurred in osseocutaneous grafts in the oral cavity (Visch et al. 2002).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


