and Stephanie Polites
2.1 Embryology
Understanding the embryologic processes of the human head and neck provides a foundation upon which craniofacial anatomy and related congenital anomalies could be studied. The keystone of head and neck development is the branchial arch system, analogous to the branchial apparatus that gives rise to gills for gas exchange in fish and amphibians. The human branchial arches are not used for gas exchange but instead contain cellular precursors of nearly all the structures of the head and neck. The arch system begins to develop during the 4th week, following creation of three germ layers — ectoderm, mesoderm, and endoderm — by gastrulation during the 3rd week.1 The ectoderm layer differentiates into both surface ectoderm and neural ectoderm, which is the earliest cell lineage of the human central nervous system. The term neural crest is used to describe the interface of neural and surface ectoderm, and a population of pluripotent neural crest cells is created by interactions between the two adjacent types of ectoderm. Neural crest cells migrate along predetermined pathways while dividing and differentiating into one of many possible cell types. Products of neural-crest cell differentiation include muscle, connective tissue, nervous tissue, endocrine tissue, and melanocytes. Endoderm differentiates into select visceral structures, including gut epithelium. Upon interaction with pharyngeal endoderm, predestined neural crest cells cease migration and create “bulges” of tissue together with nearby mesoderm tissue. Such collections of mesenchymal tissue constitute the earliest branchial arches.
2.1.1 Branchial arch system
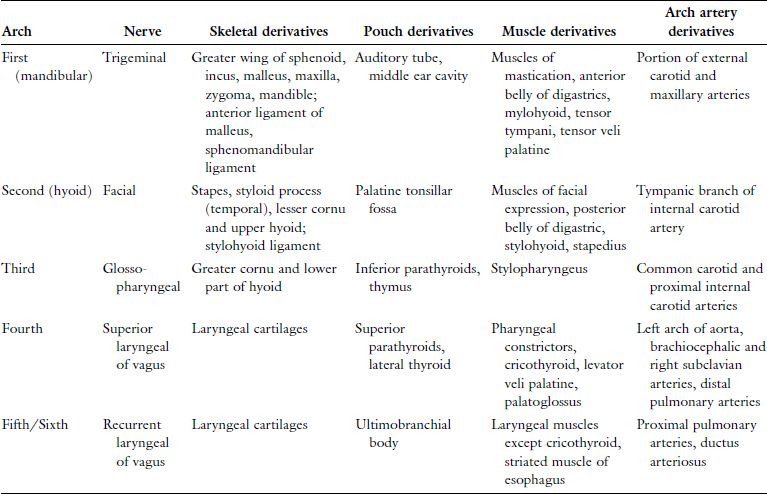
The human embryo develops a series of six paired arches where the first arch is most cranial and largest. The sixth arch is most caudal and smallest, although the fifth arch is commonly absent and considered vestigial in humans. Though derivatives of each arch are anatomically distinct, all arches are initially composed of cartilage, aortic arch artery, muscle, and nerve elements (Figure 2.1). The cartilaginous rod of each arch gives rise to bone, cartilage, and ligament derivatives of that arch. It is important to note that only bones formed by endochondral ossification are arch-cartilage derivatives.1 Bones that undergo membranous ossification are derivatives of arch mesoderm. The mandible, for example, is derived from the first arch and has both cartilaginous (condyles) and mesodermal (body and ramus) origins. Additionally, only the first and second arches have ligamentous derivatives. The six aortic arch arteries serve to connect the embryonic heart, located ventral to the endoderm-lined pharynx, and the dorsal primitive aorta. The aortic arch arteries are initially named by their associated arch number but later contribute to arteries of the mature human circulation. The nervous tissue component of each arch gives rise to both motor and sensory fibers of a cranial nerve that is then associated with that arch. All human muscle development relies on adequate innervation to initiate formation of myoblasts from mesoderm. Therefore, the mesoderm-derived muscle component of each arch develops into muscles that retain motor innervation by the cranial nerve associated with that arch.
The branchial arch system consists of multiple tissue layers that essentially recapitulate the trilaminar disk formed by gastrulation. The term branchial pouch is used to describe the internal endoderm lining of the invaginated interface, or cleft, between adjacent arches. Branchial grooves are corresponding ectoderm-lined clefts on the external surface of the arch system. The ectoderm and endoderm of the grooves and pouches, respectively, also give rise to structures of the head and neck (Figure 2.2). Although there are anatomically identifiable derivatives of all branchial pouches, all but the first branchial groove are obliterated by overgrowth of the second groove. As it grows caudally, the second branchial groove creates an ectoderm-lined cervical sinus that is sealed to the outside by the 6th week and eliminated by the 7th week. Persistence of the cervical sinus is a congenital anomaly that results in a branchial fistula, sinus, or cyst.1 A patent cervical sinus at birth is classified as a branchial fistula if the sinus communicates with the pharynx, a branchial sinus if communicating with the surface of the skin of the neck, and a branchial cyst if the patent cervical sinus is contained within the neck. The remaining first branchial groove forms the external auditory meatus as well as the external lining of the tympanic membrane, which consists of a thin layer of mesoderm interposed between a layer of endoderm from the first pouch and ectoderm of the first groove.
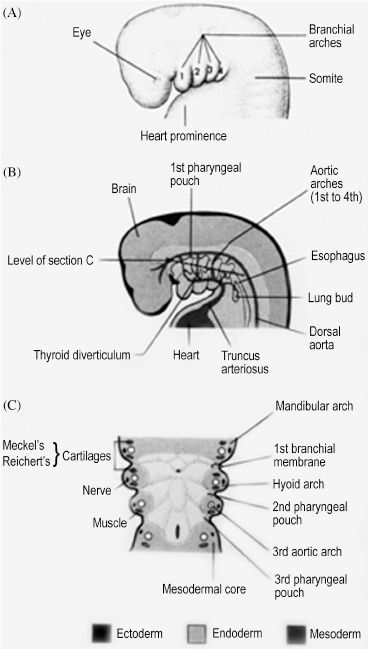
Figure 2.1. Each branchial arch has a cartilage, nerve, muscle, and aortic arch component.1 Reprinted with permission from Wolters Kluwer (Ref. 1; p. 180).
The branchial pouches can be divided into dorsal and ventral, as each area can give rise to different derivatives (Table 2.1). The ventral portions of the first and second pouches are obliterated by growth of the tongue. The dorsal first pouch forms the embryonic tubotympanic recess, which becomes the auditory tube and middle ear cavity of the human ear. The lateral surface of the tubotympanic recess forms the internal layer of the tympanic membrane, as discussed previously. The dorsal second pouch forms the tissue bed upon which the future palatine tonsils rest, called the palatine tonsillar fossa. Dorsal endoderm of the third and fourth pouches becomes the inferior and superior parathyroid glands, respectively. This counterintuitive process indicates that significant caudal migration of the inferior parathyroid glands occurs. As thymus tissue, derived from ventral endoderm of the third pouch, travels to the superior mediastinum, it effectively pulls the parathyroid tissue of the third pouch caudal to the parathyroid tissue of the fourth pouch.2 Lastly, endoderm of the fifth branchial pouch forms the ultimobranchial body, from which parafollicular C cells originate. These cells become interspersed in the follicular epithelium of the thyroid gland and secrete calcitonin, a hormone with a minor role in calcium homeostasis.
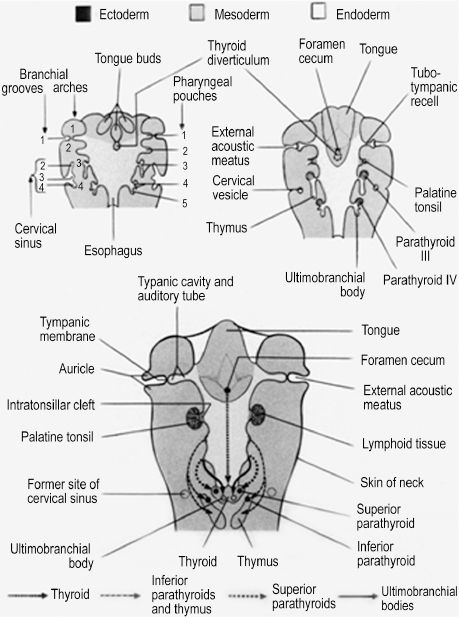
Figure 2.2. The internal endodermal surface and external ectoderm surface of the branchial apparatus gives rise to structures and organs of the head and neck.1 Reprinted with permission from Wolters Kluwer (Ref. 1; 182).
Table 2.1. Summary of the Branchial Apparatus
2.1.2 Development of human facial features
Though the entire branchial arch system contributes to structures of the head and neck, the first branchial arch and mesenchyme ventral to the forebrain are responsible for development of the features characteristic of the human face (Figure 2.3).3 During the 4th week, the primitive mouth, or stomodeum, is the first recognizable feature to appear. Initially it is formed by a central collection of mesenchyme ventral to the forebrain, known as the frontonasal prominence, and the paired cartilaginous maxillary and mandibular prominences of the first arch. The cephalic border of the stomodeum is formed by the frontonasal prominence, the lateral borders by the paired maxillary prominences, and the caudal border by the paired mandibular prominences. While the incus and greater wing of the sphenoid form from endochondral ossification of maxillary cartilage, the actual maxilla, zygoma, and squamous temporal bone form by membranous ossification within the maxillary prominence. Likewise, cartilage within the mandibular prominence forms only the malleolus of the mandible and serves as a template for membranous ossification of the body and ramus. The caudal face is the first region to take recognizable human form, as the paired mandibular prominences migrate and fuse medially early in the 4th week, forming the primitive chin, lower lip, lower jaw, and lower cheek. Migration and fusion of the primary facial prominences occurs largely from weeks 4 to 8, and an embryo has a distinctly human face by week 10.
By the end of the 4th week, nasal development begins with appearance of bilateral nasal placodes at the lateral, inferior surface of the frontonasal prominence (Figure 2.3). In human embryology, the term placode refers to thickening of surface ectoderm. As the nasal placodes enlarge, they develop distinct medial and lateral regions, which are termed medial and lateral nasal prominences. The central depressed regions of each placode, or nasal pits, are the earliest manifestations of nares. The bilateral nasal pits lie between their respective medial and lateral nasal prominences and are initially in contact with the stomodeum. This continuity is obliterated due to central migration of the paired maxillary prominences and bilateral medial and lateral nasal prominences.1 This important migratory process creates a single nose, upper lip, and primary palate. Specifically, medial fusion of the medial nasal prominences creates the nasal septum, nasal tip, and philtrum of the lip. The lateral nasal prominences develop into the nasal alae. Fusion of the paired maxillary prominences forms the upper lip (excluding the philtrum), upper jaw, and upper cheek. The primary palate is created by fusion of the maxillary prominences with the medial nasal prominences. Failure of this fusion can result in a primary cleft palate.4 The lateral nasal prominences are separated from the maxillary prominences by bilateral furrows in the mesenchyme, termed the nasolacrimal grooves. The grooves epithelialize and become sealed from the facial surface, forming paired nasolacrimal ducts. These ducts become patent after birth, to drain lacrimal gland secretions from the medial conjunctival sacs to the nares. The maxillary prominences also fuse with the mandibular prominences, creating the lateral commissure of the mouth.
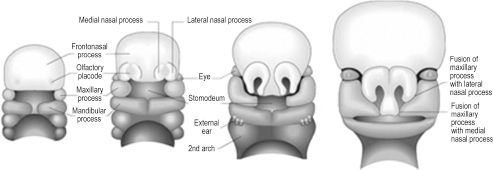
Figure 2.3. Human facial features develop from the paired maxillary and mandibular processes and the unpaired frontonasal process.3 Reprinted with permission from Elsevier (Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 39th ed. Chapter 34, 2005:609). Copyright © 2005 Elsevier.
The actual nasal cavities are created when the nasal pits deepen to form nasal sacs that are separated from the oral cavity by the oronasal membrane. This membrane ruptures, allowing communication between the oral and nasal cavities just posterior to the primary palate via primitive choanae. Upon formation of the secondary palate by fusion of the lateral palatine processes and nasal septum, the choanae are shifted posteriorly. In their final position, the choanae allow communication between the oral cavity and the pharynx. The lateral walls of each nasal cavity give rise to three elevations that become the superior, middle, and inferior conchae.
The nasal cavities are also responsible for creation of the paranasal air sinuses, which are created when diverticula of the lateral walls grow into the sphenoid, ethmoid, frontal, and maxillary bones. The maxillary sinuses are the first to develop, appearing in the 3rd fetal month, followed by the ethmoid sinuses in the 5th month. The sphenoid sinus appears at 5 months postnatally and the frontal sinuses between 5 and 6 years of life. The olfactory epithelium responsible for the sense of smell is derived from ectoderm in the roof of each nasal cavity. This epithelium is composed of several cell types that include olfactory cells, which are actually bipolar neurons of the olfactory nerve responsible for odor detection.5
In addition to forming the forehead, the frontonasal prominence is involved in early development of the eyes. As mentioned previously, placodes are formed by thickening of surface ectoderm. This process occurs within the lateral facets of the frontonasal prominence, forming bilateral lens placodes. Surface ectoderm is also notably responsible for the eyelids, which appear during the 8th week and overgrow the developed eyes. The lens placodes are induced to fold inwards by outgrowths of the forebrain, the optic stalks, thereby forming lens vesicles once the placodes lose contact with the surface (Figure 2.4). The optic vesicles are hollow bulbs enclosing the developing eyes at the distal ends of the optic stalks. The lens vesicles continue to differentiate into the lenses, the optic stalks into the optic nerves, and the optic vesicles into the optic cups. Though the optic nerve is also the second cranial nerve, its embryologic development indicates that it is actually a part of the central nervous system. Neural crest cells soon populate the region to become the sclera and choroid just superficial to the cup and the ciliary bodies at the medial and lateral margins of each cup. The cornea is also derived from neural crest cells superficial to the lens. Though the eyes are initially placed eccentrically on the face, they undergo accelerated medial migration between the 5th and 9th week.
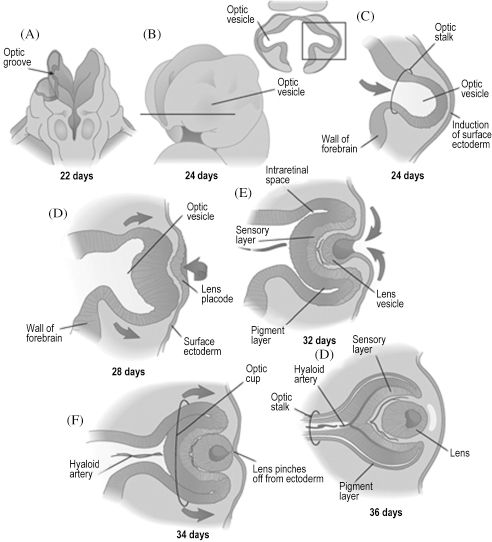
Figure 2.4. The eye begins as an evagination of the forebrain, which induces overlying ectoderm to become the lens.6 Reprinted with permission from Elsevier (Ref. 6; p. 293). Copyright © 2004 Elsevier.
Development of the human tongue is initiated during the 4th week, when the median tongue bud appears from the floor of the pharynx. Subsequent development can be divided into that of the anterior two-thirds (oral) and posterior one-third (pharyngeal) tongue. The terminal sulcus is a V-shaped furrow visible in the adult tongue that superficially divides anterior from posterior tongue. The oral tongue is formed when two lateral lingual swellings overgrow the median tongue bud. Both the median tongue bud and lateral lingual swellings, also known as distal tongue buds, are derived from first-arch mesenchyme. The posterior tongue is initially formed by mesenchyme of the second arch, or the copula. The copula is soon overgrown by the hypobranchial eminence of the third and fourth arches. Innervation of the tongue is rooted in its embryologic origins, as the oral tongue receives sensory innervation from the mandibular branch of the trigeminal nerve and the pharyngeal tongue from the glossopharyngeal and superior laryngeal nerves. Only the muscular components of the tongue are not derived from the branchial arches, originating instead from occipital somites. All tongue muscles except the palato-glossus, which is innervated by the vagus nerve, receive motor innervation from the hypoglossal nerve. The dorsum of the tongue has a midline depression called the foramen cecum (Figure 2.5).
Also during the 4th week, endoderm of the foramen cecum proliferates to create the thyroid diverticulum. As this diverticulum descends within the neck, anterior to the hyoid bone, laryngeal cartilages, and trachea, it remains connected to the tongue via the thyroglossal duct. The diverticulum completes its descent when it reaches the second and third tracheal rings, usually by the 7th week. It solidifies and takes its adult form of bilateral lobes connected by a central isthmus. Asymptomatic anomalies of thyroid development include undescended thyroid tissue at the foramen cecum, known as a lingual thyroid, and persistence of thyroid tissue in the distal thyroglossal duct, forming a third lobe of the thyroid gland.6
The human palate is composed of a primary and secondary palate, each with distinct embryologic origins and processes.4 The incisive foramen just posterior to the maxillary incisors indicates the intersection of primary and secondary palates in the adult palate. The primary palate is derived from the frontonasal prominence via the median palatine process, which forms upon the merging of the medial nasal prominences. The secondary palate is formed from the lateral palatine processes, which are bilateral outgrowths of the maxillary prominences. The lateral palatine processes are initially oriented vertically and evolve to the horizontal position during the 8th week, allowing medial migration. The developing tongue is interposed between the lateral palatine processes in their vertical stage, and micrognathia by pushing the tongue cephalad can delay or prevent the horizontal shift. Eventual midline fusion of the processes and creation of a single mesenchymal secondary palate depends on apoptosis of the epithelial cells lining the medial shelves during migration. The line of fusion, called the palatine raphe, can be seen in the adult palate (Figure 2.6). The palate is complete only upon fusion of mesenchyme of the medial and lateral palatine processes as well as fusion of the lateral palatine processes with the nasal septum. If any of the aforementioned steps are disrupted, preventing complete coalescence of appropriate mesenchymal processes, a cleft palate results. The embryology of the palate is clinically relevant, as the location and degree of clefting is directly related to the stage of development that was disrupted. The hard palate is named as such due to ossification of the primary and anterior secondary palates. The posterior portion of the secondary palate does not ossify and becomes the soft palate.
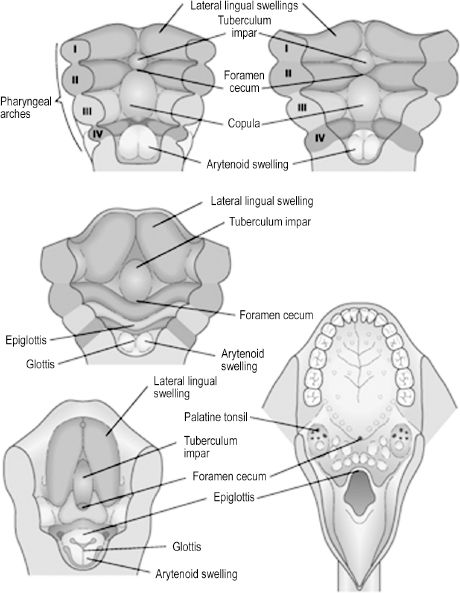
Figure 2.5. The anterior tongue is formed by derivatives of the first arch and the posterior tongue by derivatives of the third arch.6 Reprinted with permission from Elsevier (Ref. 6; p. 344). Copyright © 2004 Elsevier.
The external ear is composed of the auricle and the external acoustic meatus. Like most other elements of facial anatomy, the external ear is derived from the branchial arch system. The first branchial groove lies between the first and second arches, and the auricle is created on its dorsal aspect by means of six mesenchymal swellings, or auricular hillocks. The caudal region of the first arch contributes three anterior hillocks, and three posterior hillocks arise from the cephalic portion of the second arch. The hillocks undergo progressive expansion and fusion to create a recognizable auricle by the end of the 8th week (Figure 2.7). Cutaneous sensation of the auricle recapitulates its embryology, as portions derived from first-arch hillocks are innervated by the trigeminal nerve (auriculotemporal branch) and portions from second-arch hillocks by the facial nerve and cervical plexus (great auricular nerve). The external auditory meatus is created during the 2nd month, when ectoderm of the dorsal region of the first branchial cleft grows inward. Initially, the meatus is occluded by a proliferation of ectoderm known as the meatal plug. This plug canalizes late in the 7th month of development. As the auricles and external auditory meatuses develop, they shift cranially and dorsally from the neck region to the lateral face. This is clinically significant because any aberration or arrest of branchial arch development results in caudally displaced auricles at birth.
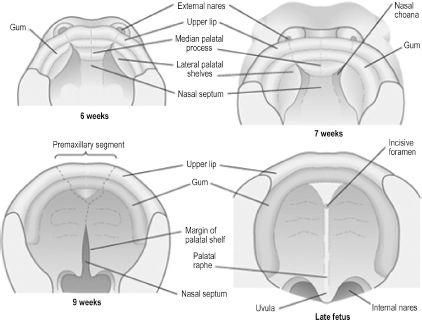
Figure 2.6. Development of the primary and secondary palate.6 Reprinted with permission from Elsevier (Ref. 6; p. 326). Copyright © 2004 Elsevier.
Development of the cranium, or skull, can be divided into that of the neurocranium and viscerocranium. The neurocranium is the skeletal encasement of the brain and the viscerocranium is the facial skeleton. The neurocranium can further be divided into the skull base and the cranial vault. The skull base is termed the chondrocranium, as it is derived from cartilages that undergo endochondral ossification (Figure 2.8). Neural crest cells give rise to cartilages of the chondrocranium that lie rostral to the sella turcica. Cartilages posterior to this landmark originate from paraxial mesoderm via occipital somites. Bones of the cranial vault, also known as flat bones, undergo membranous ossification, meaning that neural crest cell and paraxial mesoderm mesenchyme ossify directly without a cartilaginous stage. Development of the cranial vault is not complete at birth, as the flat bones are separated by connective tissue sutures. The term fontanelle is used to describe the connective tissue intersection of three or more bones. These flexible components of the cranial vault are necessary to allow shifting and overlap of the bones during child-birth and to accommodate the brain as it grows rapidly throughout childhood. The viscerocranium is also formed by both endochondral and membranous ossification of branchial arch contributions. Bones of the facial skeleton that undergo endochondral ossification are derived from neural crest cells that become arch cartilages, while bones undergoing membranous ossification form directly from arch mesenchyme.
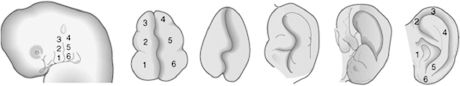
Figure 2.7. The auricle is formed by progressive growth and interaction of six mesenchymal swellings contributed by the first and second branchial arches.6 Reprinted with permission from Elsevier (Ref. 6; p. 311). Copyright © 2004 Elsevier.
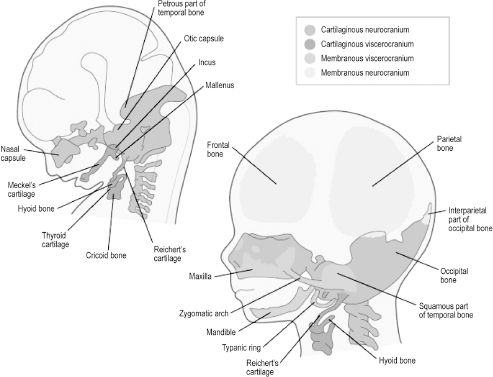
Figure 2.8. Bones of the cranial vault are derived from membranous neurocranium, whereas those of the skull base are from cartilaginous neurocranium. Facial bones are derived from membranous or cartilaginous viscerocranium.6 Reprinted with permission from Elsevier (Ref. 6; p. 192). Copyright © 2004 Elsevier.
2.2 Bones
2.2.1 The cranial vault
The entire neurocranium is constructed from six bones. The frontal bone, paired parietal and temporal bones, and the occipital bone form a cranial vault with a concave interior facade to encase the convex surface of the brain (Figure 2.9).7 Since the frontal bone is the most anterior bone of the cranial vault, it is also important in facial anatomy, forming the forehead and roof of the orbit. The frontal bone is fused posteriorly to the paired parietal bones at the coronal suture. The parietal bones form the superior and lateral surfaces of the cranial vault, meeting in the midline at the sagittal suture and meeting the unpaired occipital bone posteriorly at the transverse lambdoid suture. The posterior fontanelle is formed by the intersection of the sagittal and lambdoid sutures. Upon ossification of the membranous fontanelle, the intersection is termed the lambda. Likewise, the anterior fontanelle is the membranous intersection of the coronal and sagittal sutures, becoming the bregma upon ossification. The vertex, or highest point, of the skull is usually found approximately halfway along the sagittal suture as it runs from anterior to posterior. The superior and inferior temporal lines arch across the exterior surface of the parietal bones. The former gives rise to the temporal fascia and the latter to the temporalis muscle. At their inferior edge, the parietal bones articulate with the superior aspect of the paired temporal bones, creating bilateral squamosal sutures.
An important bony landmark of the lateral cranium is the pterion, which marks the intersection of the frontal, parietal, temporal, and sphenoid bones. The temporal bone is divided into several regions, the squamous portion, temporal portion, mastoid process, and petrous portion, each representing a separate center of ossification. The squamous portion, or squama temporalis, represents the temporal bone’s contribution to the cranial vault and gives rise to the zygomatic process. The squama also contains the mandibular fossa and the petrotympanic fissure. The former is a depression housing the mandibular condyles at the temporomandibular joint (TMJ) and the latter an opening containing the chorda tympani from the middle ear to the mandibular fossa, where it exits the skull. The tympanic portion of the temporal bone lies just inferior to the squama and anterior to the mastoid process. It blends medially with the petrous temporal bone and is attached laterally to the cartilaginous part of the external acoustic meatus. Only the outer one-third of the meatus is formed by cartilage; the inner two-thirds are formed by the tympanic portion of the temporal bone.
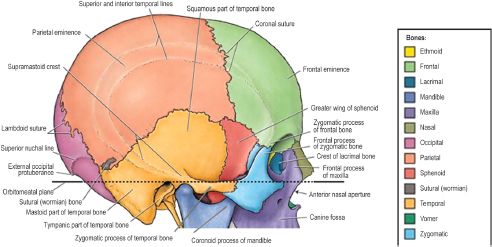
Figure 2.9. The cranial vault is a dome-shaped bony enclosure for the brain. It consists of the frontal, parietal, temporal, occipital, and sphenoid (greater wing) bones, which articulate at anatomically identifiable suture lines.7 Reprinted with permission from Wolters Kluwer (Agur AMR, Dalley AF II, eds. Grant’s Atlas of Anatomy. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:613). Copyright © 2009 Lippincott Williams & Wilkins.
Just posterior to the opening of the external acoustic meatus is the mastoid process, which does not appear until after birth. This conical process extends inferiorly from the posterior temporal bone and completes development only at puberty, when it is filled with mastoid air cells. The motor branch of the facial nerve exits the skull through the stylomastoid foramen, just medial to the mastoid process, and is vulnerable to injury during childbirth and in infancy when the mastoid process has not developed. The tympanic portion also gives rise to the styloid process, a horn-shaped piece of bone extending inferiorly from the temporal bone anteromedial to the mastoid process. This process provides attachment for several muscles and ligaments of the head and neck.5
2.2.2 External skull base
The base of the cranium is formed by the inferior neurocranium, including the occipital, temporal, and sphenoid bones, and the viscerocranium excluding the mandible (Figure 2.10). The most anterior aspect of the external base of the cranium is formed by the maxilla. It communicates laterally with the zygomatic bone and posteriorly with the horizontal plates of the palatine bones. Superior to the posterior free edge of the hard palate is the posterior nasal apertures, or choanae, separated by the vertically oriented vomer bone. The vomer articulates inferiorly with the hard palate and posteriorly with the unpaired sphenoid bone. The sphenoid bone comprises a body and three sets of paired processes — the greater wings, lesser wings, and pterygoid processes. Each pterygoid process extends inferior and perpendicular to the cranial base, separating distally into a medial and lateral pterygoid plate. The latter allows attachment of the medial and lateral pterygoid muscles, two muscles of mastication. The greater wings articulate posteriorly with the petrous portion of the temporal bone, and there is a groove in the inferior aspect of the synchondrosis for the cartilaginous segment of the auditory (eustachian) tube. The posterior portion of the cranial base is formed by the occipital bone.
Visible on the external aspect of the occipital part of the cranial base is the foramen magnum, a large opening through which the spinal cord and its meninges pass, along with the vertebral arteries, spinal arteries, and accessory nerve. The skull articulates with the vertebral column at paired occipital condyles that lie on either side of the foramen magnum. The jugular foramen is formed by a gap in the articulation between the occipital and petrous temporal bones. It contains the origin of the internal jugular vein from the sigmoid sinus and three cranial nerves: the glossopharyngeal, vagus, and accessory nerves.
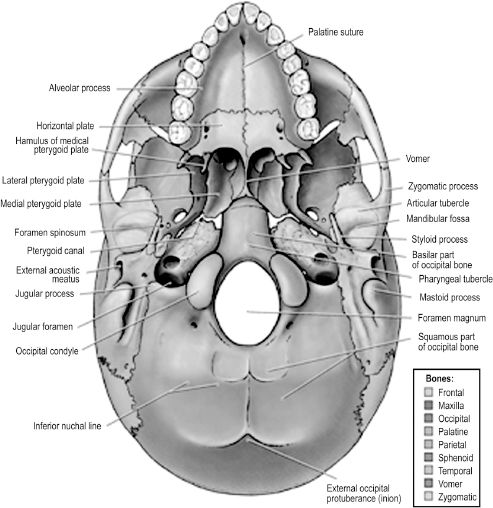
Figure 2.10. The external base of the skull includes bones of the facial skeleton except for the mandible, and transmits many intracranial neural and vascular structures to the face and neck.7 Reprinted with permission from Wolters Kluwer (Agur AMR, Dalley AF II, eds. Grant’s Atlas of Anatomy. 12th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:617). Copyright © 2009 Lippincott Williams & Wilkins.
There are several bony landmarks visible on the occipital portion of the external cranial base. The external occipital protuberance is a midline elevation that may be externally palpated on the human head. The superior and inferior nuchal lines are transverse elevations that provide attachment to muscles of the neck and the occipital body of the occipitofrontalis.
2.2.3 Internal skull base
The internal, or superior, face of the skull base can be divided into three spaces — the anterior, middle, and posterior cranial fossae (Figure 2.11). The anterior fossa comprises the frontal bone, the superior ethmoid bone, and the lesser wings of the sphenoid bone. The floor of the frontal bone contains depressions formed by ridges in the overlying frontal bone of the brain in addition to a hiatus, termed the ethmoidal notch, for the crista galli and cribriform plate of the ethmoid bone. The crista galli is a midline, upward projection from the ethmoid bone surrounded on either side by the porous cribriform plate. Axons composing the olfactory nerve pass through the cribriform plate, terminating in the olfactory bulb that lies atop this part of the ethmoid bone. The anterior midline of the frontal bone contains a protuberance, or frontal crest, that is formed by the underlying superior sagittal sinus. The foramen cecum is a small opening at the base of the crest that is usually closed in the adult but patent during fetal development. It is clinically significant because it may permit passage of a nasal emissary vein to the superior sagittal sinus, providing a route for cutaneous facial infection to reach the cranial cavity. The anterior cranial fossa is separated from the middle fossa by the sharp posterior edges of the lesser wings of the sphenoid (sphenoidal crests) and the sphenoidal limbus that lies between two anterior clinoid processes. Also of note is that the crista galli and frontal crest provide attachment for the falx cerebri and the anterior clinoid processes for the tentorium cerebelli.
The middle cranial fossa is formed by portions of the sphenoid and temporal bones. Centrally, the fossa contains the sella turcica atop the body of the sphenoid bone. The greater wings of the sphenoid, the petrous temporal, and the squama temporalis bones are found on either side of the sella turcica, forming the lateral areas of the middle fossa. The fossa is bounded anteriorly by the lesser wings of the sphenoid and anterior clinoid processes as well as the chiasmatic groove, a transverse depression in the anterior body of the sphenoid for the optic chiasm. The groove leads into bilateral optic foramina on either side that transmits the optic nerves and ophthalmic arteries to each orbit. The posterior boundaries of the middle cranial fossa are the petrous crests laterally and the dorsum sellae medially. The former are elevations in the petrous temporal bones and the latter a single vertically orientated plate of bone that terminates bilaterally in the posterior clinoid processes. The sella turcica is a saddle-shaped structure formed by the superior surface of the body of the sphenoid. The “seat” of the saddle is a depression in the body of the sphenoid that is occupied by the hypophyseal fossa, or space for the pituitary gland. The sphenoid sinus, which drains to the posterior nasal cavity, is found in the body of the sphenoid just deep to the sella. Further clinical significance of the middle cranial fossa is attributed to multiple openings in the greater wing of the sphenoid that allow passage of nerves and vessels from the intracranial cavity (Table 2.2).
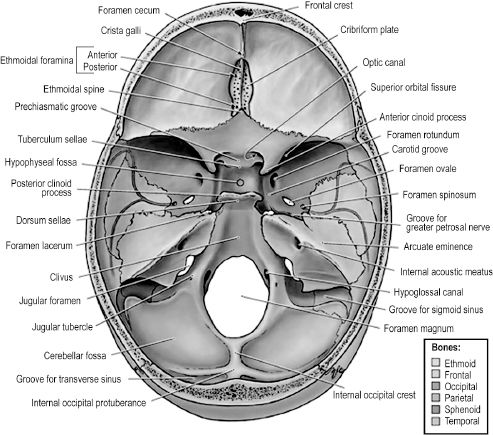
Figure 2.11.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


