Fig. 6.1
Draft representing the typical irradiation proposed for conventional laser endodontics; the fiber is positioned in a dry canal 1 mm shorter from the apex and is retracted with helical motion in an established time (2 mm/s) (Image courtesy of Giovanni Olivi, Rome, Italy)
The technique requires helical (circular) movement of the fiber, in order to increase the irradiation angle between laser fiber and dentin surface, trying to improve the angle (directionality) and energy diffusion.
This technique is suitable for most wavelengths used in dentistry, in the visible (532 nm), in the near-infrared (from 810 to 1340 nm), and medium-infrared (2780 and 2940 nm) electromagnetic spectrum and utilizes fibers or tips (flat or end firing), which permit the direct irradiation of the canal surface.
Olivi (2013) called this technique “conventional laser endodontics” (see Table 5.1) [1], in order to distinguish it from the more recent PAD (or aPDT) and LAI, which involve the irradiation and the activation of different antibacterial solutions and irrigants.
6.1 Mechanism of Conventional Laser Endodontics
When a laser fiber/tip is inserted into the canal and laser irradiation starts, it interacts with the canal surface following the optical characteristic of every wavelength (see Sect. 5.4).
The presence and concentration of specific chromophores on the canal surface and inside the dentinal tubules determine absorption, diffusion, or transmission of laser light. Depending on the wavelength, there are different effects and different depths of penetration (see Sect. 5.4).
Under the same conditions, the medium-infrared wavelengths, which are well absorbed by the dentin water (chromophore), spread their energy superficially over the canal surface, while the near-infrared wavelengths, which are not well absorbed by the dentin chromophore, are more penetrating in depth.
If the objective is removal of tissue (ablation-preparation of the canal), correct management of the wavelength and parameters (energy, power, and pulse duration) allows superficial absorption, reducing heat diffusion which is inefficient for ablative purposes. On the contrary, if the objective is not ablation but decontamination, the ability for penetration through the target tissue of the selected wavelength can constitute a desirable and valued advantage.
The medium-infrared laser radiation is mainly absorbed at the canal surface by the watery component of dentin, with vaporization of debris and smear layer, leaving the dentinal tubules open and also producing surface decontamination and minimal dental ablation.
The near-infrared laser radiation mainly diffuses in the dentinal tubules and dentin walls, to target the pigment of bacterial cells performing a deep decontaminating effect and also a thermal effect on the surface during the diffusion of light; the thermal effect results in inorganic melting of the smear layer producing occlusion of dentin tubules, more or less evident depending on the wavelength and the parameters used (fluence, peak power, and modality of emission).
When the energy applied is high, many undesired thermal effects on the dentin walls are present, varying on the wavelength used.
Laser technology has been proposed for different phases of endodontic treatment for:
-
Access cavity preparation
-
Root canal preparation
-
Root canal cleaning
-
Root canal decontamination
Research and clinical use, after initial interest in all possible therapeutic uses of lasers in endodontics, are today limited to cleaning and decontamination of the root canal system.
6.2 Access Cavity
Beyond the conventional use of rotative instruments, the access to the pulp chamber can be performed with erbium lasers, able to remove all the hard and soft dental tissues: enamel, dentin, carious tissue, and pulp without contact. These lasers can be used both with a handpiece with terminal tip and with a tipless handpiece. When using a handpiece with a tip, the choice of diameters ranging from 600 to 1000 microns is preferable. Larger diameters give rise to more elevated energy and power during ablation of the enamel with a larger irradiated surface. Preparing the access cavity, the energy is progressively reduced in depth, from the dentin to the pulp. The high affinity with carious tissue and pulp (richer in water) helps to remove carious dentin and to selectively uncover the pulp horns with less energy, reducing the risk of a false path. The following are the suggested energy setting to progressively use on enamel, dentin, carious tissue, and pulp:
|
Enamel
|
Dentin caries
|
Pulp tissue
|
Canal orifices
|
|
250 mJ>
|
200 mJ >150 mJ
|
150 mJ
|
120 >80 mJ
|
The importance of the laser approach for access cavity preparation should not be underestimated. Erbium lasers, due to their selectivity of action, allow an access to the endodontic system after a consistent abatement of bacterial load, therefore reducing contamination with bacteria, toxins, and debris in the apical direction during subsequent instrumentation. A study by Hibst et al. (1996) demonstrated that bacteria are killed during cavity preparation up to a depth of 300–400 microns, under the irradiated surface [2].
Erbium lasers are also useful in the removal of pulp stones and in the search for calcified canals.
6.3 Lasers for Root Canal Preparation
Root canal preparation with steel and nickel-titanium instruments is today considered the gold standard in Endodontics. This section is presented with the sole aim to give information on the development of laser technology in Endodontics.
6.3.1 Excimer Laser
Among the first experiments with lasers in endodontics, root canal preparation was studied in vitro by Liesenhoff et al. (1989), which used an excimer laser (308 nm) and concluded that the laser can prepare a root canal safely and efficiently and produce a canal surface without a smear layer, with open dentinal tubules and a lack of over-instrumentation and false path (via falsa) [3].
In another in vitro study, Frentzen et al. (1991) reported opposite results, concluding that the energy density of excimer laser was not high enough for the ablation of dentin and pulp tissues [4].
The value of excimer laser technology in endodontics was limited. The latter also accounts for their use in dentistry in general.
6.3.2 Nd:YAG Laser
At the beginning of the 1990s, the first investigations with Nd:YAG laser with optical fiber in the root canals were started.
Gutknecht et al. (1991), in an in vitro preliminary study with SEM observation, showed the modifications of the root canal surface following irradiation at 1.5 W (100 mJ, 15 Hz); the smear layer was removed through superficial inorganic melting with closure of dentin tubules, and these modifications were considered useful for endodontic treatment [5].
Levy (1992) as well, in a SEM study, concluded that Nd:YAG laser preparation was possible and that it allowed better cleaning of canal surface than conventional instrumentation [6].
Goodis et al. (1993) confirmed the Nd:YAG laser’s ability to interact with dentin walls with smear layer removal and, only occasionally, surface alterations were seen [7].
Some years later, Moogi and Nageshwar (2010) reported the same results. SEM observations showed the presence of areas of fusion and recrystallization, especially in the apical one-third; these dentin surface alterations were positively considered by the authors, as a possible seal to bacterial recolonization [8].
Research on the use of Nd:YAG technology for root canal preparation was soon abandoned and the technology is today used in endodontics only for the final decontamination at the end of conventional chemomechanical instrumentation.
6.3.3 Nd:YAP Laser
Blum and Abadie (1997) compared different systems of root canal preparation, using sodium hypochlorite as irrigant. Manual preparation and combined manual/laser-assisted techniques ended in similar results, differing just in size of remaining debris, that were smaller in case of laser-assisted preparation. Preparation executed with just laser irradiation produces an enhanced diameter in the prepared canals with the presence of debris. Different conventional manual preparations, associated with both laser irradiation and subsonic irrigation, showed no statistically significant differences. The combined use of subsonic and laser systems, associated with manual preparation, demonstrated better cleaning after preparation, with very small debris remnants. The study concluded by referring to the potential capacity of Nd:YAP laser to complete manual preparation of the canal with better cleaning [9].
Also for Nd:YAP laser, studies on this specific use were not continued.
6.3.4 Erbium Lasers
The recognized ablative capacity on hard tissues of erbium lasers (2780 and 2940 nm), and their FDA approval (according to the US FDA Marketing Clearance by Wavelength) for shaping, enlarging, and cleaning of the canals, guided the following research toward medium-infrared lasers.
Many studies analyzed the erbium lasers’ potential for root canal preparation in different operative conditions, evaluating the efficacy, possible morphological damage, and possible thermal damage to the periodontal tissues.
The initial studies reported positive results using Er:YAG laser with different experimental radial emission tips, encouraging future research.
Shoji et al. (2000) used a Er:YAG laser system with a conical tip with 80 % lateral emission and 20 % end emission, for the enlargement and cleansing of the canals.
Using 0.3 W (30 mJ and 10 pps), they obtained canal enlargement of about 13 % with a cleaner dentinal surface compared to the samples treated without laser [10].
Kesler et al. (2002), in a preliminary study on the effects of Er:YAG laser, equipped with microprobes with radial emission, used 140 and 90 mJ with 400 and 200 micron tips, respectively, and reported, in the study’s conditions, good capacity for enlargement and shaping, quickly and better than the traditional system. The SEM observations demonstrated a dentin surface equally clean from the apex to the coronal portion, without pulp remnants and clean open dentinal tubules [11].
Stabholz et al. (2003 and 2004) also presented positive results regarding treatment executed with the same Er:YAG laser and lateral emitting endodontic microprobes [12, 13].
A study by Inamoto et al. (2009) investigated in vitro the cutting capacity and morphological effects of Er:YAG laser irradiation, using 0.3 and 0.75 W (30 mJ at 10 Hz and 25 Hz), withdrawing a fiber at a speed of 1 mm/sec and 2 mm/sec, reporting positive results [14].
Recently, Kokuzawa et al. (2012) evaluated the shaping capacity of the root canal using Er:YAG laser equipped with conical fibers of 185 and 280 micron diameter; the conical fibers diffused 80 % of the energy laterally. Irradiation was executed at 0.4 W (20 mJ, 20 pps), with water spray (5 ml/min), 3 times for 10 s. SEM observations showed clean dentin surface, with open tubule orifices. The authors underlined that for efficient ablation, a shorter distance between the fiber and the walls of the canal is necessary, in order to avoid natural dispersion of energy due to the distance between source and target [15]. This observation emphasizes the difficulty to apply the concept of fluence in endodontics that will be debated in the discussion session. Also, the difference of energy settings used in these studies underlines the different technologies of the laser systems used and the impossibility to establish a common protocol.
Studies on radial firing tips were resumed again by other investigators [16, 17] as a prelude to techniques of laser activation of the irrigants (LAI). The latter will be addressed later.
Other studies instead investigated Er, Cr:YSGG and Er:YAG lasers, equipped with plane end-firing tips.
Chen (2002 and 2003) presented case studies with root canals entirely prepared using the Er,Cr:YSGG laser (the first to obtain the FDA license for the entire endodontic procedure) with positive results. Chen used endodontic end-firing tips of different lengths, with diameters of 400, 320, and 200 microns, and used with a crown-down technique at 1.5 W (75 mJ and 20 Hz) with 35–25 % water spray [18, 19].
A study by Minas et al. (2010) showed positive results using Er,Cr:YSGG laser at 20 Hz and different power settings of 1.5, 1.75, and 2,0 W and higher water spray (65 % water, 35 % air). The study concluded that the laser’s ablative capacity proved to be strongly influenced by the power and the diameter of the fiber used [20].
On the contrary, other studies reported less positive results regarding the use of the erbium lasers for root canal preparation of teeth with curved roots. The efficacy of laser was not comparable with the endodontic standard preparation with stainless steel manual and nickel-titanium rotary instruments.
The group at the Showa University School of Dentistry, Tokyo, Japan, performed a great body of research on the use of Er:YAG and Er,Cr:YSGG lasers, evaluating the thermal variations at periodontal level, the cleaning ability of dentin surface, and the ablative efficacy in straight and curved canals.
Kimura et al. (2002) measured temperature rise during root canal preparation with the Er:YAG laser for one minute, with a water spray at 2 Hz, 136 mJ, and 230 mJ. The temperature, measured with thermocouples, rose more at apical level (6 °C), demonstrating the safety of the procedure for the periodontal tissues at the tested parameters [21].
Ali et al. (2005), Matsuoka et al. (20051), and Jahan et al. (2006) later used a Er,Cr:YSGG laser with a power of 2 W (200 mJ and 10 Hz), with 200 and 320 micron end-firing tips. Irradiation resulted in clean dentin surfaces, with less smear layer and better cleaned surfaces as compared to conventional manual preparation. The studies concluded that the preparation of canals with curves inferior to 10 degrees can be well performed, while the preparation of canals with curves of more than 15 degrees produces ledges, perforation, and over-instrumentation [22–24].
Matsuoka et al. (20052), in another study, used the Er,Cr:YSGG laser for canal preparation with fibers of 200 and 320 micron diameter, at 2 W and 3 W and 20 Hz (100 mJ e 150 mJ, respectively), obtaining clean but irregular surfaces [25].
In 2010, Roper et al. (2010) compared conventional root canal preparation techniques with rotative instruments and laser-assisted techniques, measuring the quantity of dentin removed in the various sections of the radicular canals and the cleaning. Both techniques were equivalent in the coronal and the middle third, while the conventional technique was more efficient in the apical one-third [26].
In conclusion, the dentin surface prepared with erbium lasers is always well cleaned and without smear layer. However, as a result of laser ablation, the surface is irregular; some ledging can be present, often there are signs of superficial thermal damage, and there is a risk of root perforation or apical transposition (see Figs. 5.34, 5.35, 5.36 and 5.37). Canal shaping, realized with the erbium laser, is nowadays quite a complicated procedure, suitable only for straight and wide canals but without adding particular advantages to conventional techniques.
6.4 Morphological and Cleaning Effects of Lasers on Root Dentin Surface
Many thermographic and morphological preliminary studies were performed for identifying safe parameters of use and described the collateral effects of laser irradiation on the radicular walls. Medium-infrared lasers, even if they produce some thermal and ablative effects, clean the canal walls very well, vaporizing the smear layer, exposing the dentinal tubules, and increasing dentinal permeability [27–29].
Near-infrared lasers, on the other hand, produce more evident thermal effects without removal of dentin, with areas of recrystallization of the dentin surface where the smear layer is mainly fused, occluding the dentinal tubules [29–33].
Irradiation with KTP 532 nm laser, in the spectrum of visible light, used in dry mode at 1 W per 1 s and 5 W per 0.5 s, also led to modifications of the dentin surface and of the smear layer without modifying dentinal permeability [34].
6.4.1 Near-Infrared Laser
Many studies evaluated the thermal rise produced by near-infrared lasers and the consequent morphological alterations of the dentin walls. The smear layer is only partially removed, dentinal tubules are mainly closed as result of superficial fusion of dentin structures, and smear layer and phenomena of recrystallization and cracks are present [33, 35–38].
The effects produced by diode lasers, with different wavelengths, Nd:YAG and Nd:YAP lasers are similar but not equal at the same parameters of use, so that laser settings must be adjusted to the wavelength used. Nd:YAG laser was among the first to be studied in endodontics.
6.4.1.1 Nd:YAG Laser
Saunders et al. (1995) studied in vitro the effects of different laser power settings at 15 pps (from 0.75 to 1.7 W), with the aim of obtaining an apical seal. The study showed the removal of pulp tissue from the non-prepared canals, but there was no creation of complete dentinal fusion [39].
Harashima et al. (1997), in an in vitro study on mono-radicular teeth treated with conventional instruments, irradiated the canals with Nd:YAG laser at 1 W (50 mJ and 20 pps) and 2 W (100 mJ and 20 pps). SEM observation of the non-lased group and the irradiated group at 1 W showed canal surfaces with debris and a smear layer that covered the dentin tubule orifices. Otherwise, the group irradiated at 2 W showed cleaner canal surfaces; debris and smear layer were partially vaporized but areas of fusion and recrystallization were present [40].
Koba et al. (1998), also in vitro, investigated the effects of Nd:YAG laser irradiation, at apical and periapical level. In the majority of cases, there was a good removal of debris, correlated with the energy used. Carbonization was visible on the canal surface at 2 W, 15 pps for 2 s and at 1 W, 15 pps for 3 and 4 s, but not at 1 W, 2 s [41].
Barbakow et al. (1999) observed in vitro areas of recrystallization in the apical area at every energy setting and at 6 and 10 mm from the apex only in the group irradiated with 318 J/cm2. Areas of carbonization were also observed at 2 mm from the apex [42].
Kaitsas et al. (2001), in an in vitro study on mono-radicular teeth irradiated with Nd:YAG laser after conventional chemomechanical preparation, concluded that irradiation executed at 1.5 W (100 mJ, 15 Hz), with a fiber of 320 microns inserted at 1 mm from the apex and then withdrawn in 5 s with rotatory movement, produced a partial reduction in debris and smear layer together with the production of collateral thermal effects, such as melting, cracks, phenomena of recrystallization with bubbles, and partial obliteration of the dentin tubules [33] (Figs. 6.2, 6.3, and 6.4).


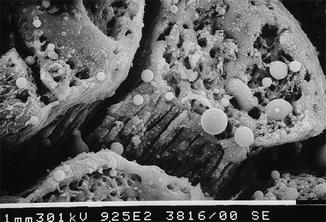

Fig. 6.2
SEM image of radicular dentin irradiated with Nd:YAG laser at 1.5 W, 100 mJ, 300 μs pulse duration, with a 200 micron fiber (2 mm/s speed). Inappropriate time of irradiation, or long pulse duration, or the contact of the fiber on dentin walls creates hot spots. The high magnification shows the dentin surface covered of melted smear layer with obliteration of dentin tubules (Imagine courtesy of Prof. V. Kaitsas Rome (Italy))

Fig. 6.3
SEM image of radicular dentin irradiated with Nd:YAG laser at 1.5 W, 100 mJ, 300 μs pulse duration, with a 200 micron fiber (2 mm/s speed). Evident melted surface with cracks and recrystallization structures resulted from laser thermal damage (Imagine courtesy of Prof. V. Kaitsas Rome (Italy))

Fig. 6.4
SEM image of radicular dentin irradiated with Nd:YAG laser at 1.5 W, 100 mJ, 300 μs pulse duration, with a 200 micron fiber (2 mm/s speed). Bubbles of recrystallization and cracks resulted from laser thermal damage (Imagine courtesy of Prof. V. Kaitsas Rome (Italy))
Santos et al. (2005) evaluated with SEM observations the effect of different parameters and angles of irradiation of Nd:YAG laser on the morphology of radicular dentin. Laser irradiation was executed at 1 W (10 Hz, 100 mJ and 20 Hz, 50 mJ) and at 3 W (10 Hz, 300 mJ and 20 Hz, 150 mJ), with a 250 micron fiber, with a retraction speed of 2 mm/s, for 1 min using a clockwise movement. More elevated parameters produced more morphological alterations of the dentin surface, with melting, glazing, recrystallization and bubbles, and superior removal of smear layer with cleaner surface. Two teeth from every group were sectioned perpendicularly and received an irradiation with perpendicular incidence. A sample received, before irradiation, a treatment with 17 % of EDTA for 5 min. The samples which received the perpendicular irradiation showed typical morphological alterations with fusion, melting, and recrystallization with bubbles. The smear layer was not removed from the dentin surface which presented a few visible tubules. The samples which received the irrigation with 17 % EDTA before irradiation showed dentinal tubules more opened and a variegated morphological pattern, depending on the energy used [43].
He et al. (2009) studied the rise in temperature at the coronal and apical levels at different power settings of a Nd:YAG laser, revealing the production of morphological thermal damage. Every sample, mono-radicular tooth, was irradiated four times for 5 s with a pause of 5 s, moving a fiber 330 micron diameter with a helicoidal movement. The most significant rise in temperature was verified at the apical level, with relevant correlation with the power and frequency of repetition used. Better results (removal of the smear layer, open dentinal tubules, rise of temperature in the acceptable limits) were obtained at 2 W (20 Hz, 100 mJ) [38].
Hasheminia et al. (2012) also confirmed the incomplete removal of the smear layer in the samples irradiated with the Nd:YAG laser and the superior efficacy of irrigants such as sodium hypochlorite and maleic acid [44].
6.4.1.2 Nd:YAP
Nd:YAP laser has a physical behavior which is slightly different from that of other near-infrared lasers, due to its major coefficient of water absorption.
Blum and Abadie (1997) underlined the usefulness of this laser in root canal cleaning [9].
Moshonov et al. (2003) also studied the capacity of the Nd:YAP laser for root canal cleaning and its influence on the mineral content of the dentin, confirming the capacity of the laser to provide cleaning statistically superior than the non-lased group, without alteration of the relationship Ca/P at dentinal level [45].
Also here, studies on this specific use were not continued.
6.4.1.3 Diode Laser
In the spectrum of the near-infrared light, diode lasers are built with different wavelengths (from 810 to 1064 nm). Their different positions on the hemoglobin absorption curve condition the adjustment of laser setting to use, but it is the lack of absorption in water that makes the interaction on the radicular dentin different.
Diode Laser 810 nm
Da Costa Ribeiro et al. (2007) studied the increase in temperature during 810 nm diode laser irradiation; at 2.5 W, the thermal rise in the apical third varied from 1.6 to 8.6 °C, while at 1.25 W, 10 Hz proved to be less, from 1.2 °C to 3.3 °C. The SEM morphological study demonstrated canal surfaces with dentinal tubules closed, especially in the apical third [36].
De Moura-Netto et al. (2005) in a study in vitro compared the effect of Nd:YAG laser (1.5 W, 100 mJ, 15 Hz) and of diode laser 810 nm (2.5 W in cw) using a fiber extraction speed of 2 mm/s, for 20 s. Both lasers proved to be able to partially remove debris and smear layer, showing also some morphological alteration (fusion and recrystallization) which was more evident with the Nd:YAG laser [46] (Figs. 6.5, 6.6, and 6.7).

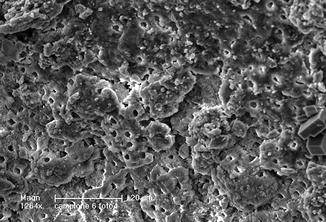
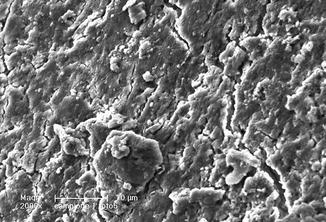

Fig. 6.5
SEM image (610×) of radicular dentin irradiated with 810 nm diode laser in dry mode at 2.5 W, 50 % duty cycle, 10 ms ton, and 200 micron fiber (2 mm/s speed). Dentin surface showing debris, melted smear layer, and few open orifices

Fig. 6.6
SEM image (1264×) of dentin irradiated with 810 nm diode laser, at 1.5 W CW, 200 micron fiber, in dry mode (2 mm/s speed). Clear signs of thermal effect are visible: surface melting with detachment of dentinal substance and partial obliteration of the dentinal tubules. The smear layer is only partially removed (Reprinted with permission from Olivi et al. [70]) (Fig. 2))

Fig. 6.7
SEM image (2000×) of radicular dentin irradiated with 810 nm diode laser in dry mode at 2.5 W, 50 % duty cycle, 10 ms ton, and 200 micron fiber (2 mm/s speed). Heavy smear layer heated by laser irradiation covering dentin surface
Parirokh et al. (2007), in their in vitro study, concluded that 808 nm diode laser produced closure of the dentinal tubules of the irradiated surface in every condition of use and especially at the level of the apical third [47].
Da Fonseca Alvarez et al. (2012) studied with thermocouple the variation of temperature on the external root surface of inferior incisors in order to evaluate possible periodontal damage. Samples were irradiated with a continuous circular retrograde movement at different parameters (1.5, 2.0, 2.5, 3.0, and 3.5 W). Thermal variations, measured on both buccal and lingual sides, at the apical level and at the middle third of the root were considered acceptable and safe within 7 °C, only the use of 3.5 W exceeded the 7 °C borderline [48].
Diode Laser 980 nm
Diode laser 980 nm was studied in vitro by Wang et al. (2005), who irradiated mono-radicular teeth for 7 s with different diameter fibers (550 and 365 micron) at 5 W power. The maximum rise in temperature was 8.1 °C. Laser irradiation produced good removal of smear layer, with cleaning statistically superior to the nonirradiated control group. Apical leakage, after the filling, also proved inferior in the laser group than in the control group [49].
The Brazilian group in the Endodontics Department of the University of Ribeirão Preto (SP, Brasile) carried out a series of in vitro studies on the 980 nm diode laser, investigating the morphological and thermal variations at radicular level with parameters from 1.5 to 5 W, in CW and gated at 100 and 1000 Hz, both dry and distilled water wet canals.
Marchesan et al. (20081) evaluated the effect on the dentinal permeability of the diode laser 980 nm after final irrigation with different solutions: distilled water, 1 % NaOCl, and 17 % EDTAC. Irradiation was performed at 1.5 and 3.0 W both in a continuous wave and gated mode (100 Hz). The laser irradiation associated with water produced an increase in dentin permeability, while a reduction in permeability was found with laser irradiation after the use of EDTAC, the conclusion being that the dentinal permeability is strictly dependent on the final irrigation solution [50].
Marchesan et al. (20082) evaluated also the morphological effect produced on the dentinal wall with the same parameters. Irradiation was executed using a helicoidal movement in exit for 20 s with different emission modes (1.5 W in CW and at 100 Hz, 3 W in CW and at 100 Hz), producing variable results from removal of the smear layer to fusion [51].
Alfredo et al. (2008) also evaluated the temperature variation using thermocouples localized at radicular and apical level, after irradiation with 980 nm diode laser at the studied parameters: 1.5, 3.0, and 5.0 W in CW and gated mode at 100 and 1000 Hz, both dry and irrigated with distilled water. The radicular portion with the major increase of temperature was the cervical third (+9.68 ± 5.80 °C), followed by the middle third (+7.66 ± 4.87 °C) and apical (+6.70 ± 4.23 °C), with a significant statistical difference between them. The continuous mode of irradiation, dry and with more power (3 W e 5 W), produced disadvantageous thermal variations (respectively +12.15 ± 5.14 °C and +7.88 ± 3.92 °C), which definitively contraindicate the use of those parameters and conditions of use. Continuous irradiation produced a more elevated thermal variation (+11.82 ±5.78 °C), whether the canal was wet or dry. Gated mode irradiation produced less thermal rise, without statistically significant differences, at 100 Hz (+6.22 ±3.64 °C) and 1000 Hz (+6.00 ±3.36 °C). The authors concluded that canal irradiation with the 980 nm diode laser at 1.5 W, in every operational mode and at 3.0 W at 100 Hz, and 1000 Hz, for 20 s, can be used safely in endodontics in both dry and wet canals [37].
In a successive study, Alfredo et al. (2009) evaluated the alteration of the canal dentin irradiated with the 980 nm diode laser at different parameters, i.e., 1.5 and 3 W in CW and gated mode at 100 Hz after irrigation with NaOCl and EDTA. It was concluded that the laser produced morphological alterations depending on the chemical pre-treatment. Pre-treatment with NaOCI showed surfaces with typical morphological alterations induced by lasers with some cracks; smear layer and closed dentinal tubules were present; those pretreated with EDTA and then irradiated presented melting areas but instead no smear layer and partially open tubules and [52]. This combined treatment is explored ahead in Sect. 6.4.6.
6.4.2 Medium-Infrared Laser
Erbium:YAG and erbium,chromium:YSGG lasers interact with the watery component of the dentin. Consequently, laser settings above the dentin threshold of ablation, the presence or not of air-water spray or of irrigants during irradiation, are all conditions that affect the morphological effects on the dentinal surface. The latter may vary from the removal of smear layer and superficial ablation to thermal damage with areas of superficial melting and creation of ledges and perforations.
Takeda et al. (1999) compared, in vitro, the effects of laser irradiation with 17 % EDTA, with that of 6 % orthophosphoric acid and that of 6 % citric acid. The effects of the CO2 and Er:YAG lasers lead to the removal of the smear layer. The study concluded that the irrigant solutions produced excessive intertubular demineralization with notable enlargement of dentin tubules. CO2 lasers on the other hand, produced fusion of the smear layer with superficial melting, while Er:YAG lasers proved to be the most efficient system for removal of the smear layer [53].
The Japanese school of the Department of Endodontics, Showa University School of Dentistry (Tokyo, Japan), conducted a great deal of research on the use of erbium:YAG and erbium,chromium:YSGG lasers.
Yamazaki et al. (2001) concluded their studies on erbium,chromium:YSGG laser, stating that the presence of water was essential to avoid undesired morphological aspects, strongly present when the irradiation was performed in dry mode. The erbium,chromium:YSGG laser, used in dry mode, besides the researched vaporization of the smear layer, produced undesired signs of ablation and thermal damage, proportionally to the power used. SEM investigation of the irradiated surface showed the presence of ledges, cracks, and melting [54].
Kimura et al. (2002) investigated the thermal rise using thermocouples located on the radicular surface after irradiation with Er:YAG lasers at 2 Hz, 136–184 mJ and 170–230 mJ for 1 minute with air-water spray. Teeth were then observed using stereo microscope and SEM. The thermal rise found at the apical level was less than 6 °C in the apical area and less than 3 °C in the radicular one-third. Morphological observations revealed the absence of carbonization or melting, using 230 mJ/pulse for 1 min [21].
When using air-water spray or in the presence of irrigant solutions, a typical pattern of dentin irradiated with the erbium laser is visible. Thermal damage is reduced; vaporization of intertubular dentin is greater than of peritubular dentine, showing a protrusion of the dentinal tubules with a cuff-like appearance; dentinal tubules are open at the peak of these peritubular areas (with more calcified dentin then for intertubular dentin and therefore less ablated). The smear layer is vaporized by irradiation with the erbium laser and mainly absent [27, 28].
Later on, Ishizaki et al. (2004) also investigated thermal rise on the periodontal surface and the morphological alterations on the dentin walls after irradiation with Er,Cr:YSGG lasers, used for smear layer removal after manual instrumentation. The Er,Cr:YSGG laser was used at 2 W (100 mJ, 20 Hz), 3 W (150 mJ, 20 Hz), and 5 W (250 mJ, 20 Hz), for 7 s with fibers of different diameters (200, 320, and 400 microns). The thermographic study showed an average thermal rise inferior to 8 °C. Observations with optical microscope showed aspects of dentin ablation at the apical terminus, while SEM indicated that the use of 5 W (250 mJ, 20 Hz), with a fiber of 400 microns, proved to be efficient in removing debris and smear layer without carbonization or melting [55].
Silva et al. (2010) investigated canal morphology and dentin permeability after Er,Cr:YSGG laser irradiation, in comparison with conventional preparation associated with EDTA-T irrigation and with conventional preparation associated with EDTA-T and irradiation with the Er,Cr:YSGG laser at different power settings: 0.75, 1.5, 2.5 W, always at 20 Hz. SEM investigation revealed irregular surfaces with morphological alterations that increased dentinal permeability, when using the laser at 1.5 W and 2.5 W [56].
Kimura et al. (2011) conducted histological examinations of the radicular surface in order to evaluate possible thermal damage after 30 s of Er:YAG laser irradiation of root canal at 2 Hz with energy of 34, 68, and 102 mJ/pulse. Irradiation with 34 mJ did not produce any thermal damage at the periodontal level, proving to be a safe parameter for irradiation. Irradiation with 68 and 102 mJ produced, in some cases, inflammation, from medium to high, with external resorption of the root surface. The difference between the control group and the laser group was not significant, with the conclusion of minimal thermal influence on the periodontal tissues, if appropriate parameters are used [57].
A study of Ebihara et al. (2012) evaluated the effects of Er:YAG laser irradiation at different parameters. The investigated fluence was 10–30 J/cm2, using a 400 micron fiber, at 30 Hz; different pulse durations (80–280 microns) with and without cooling with air-water spray were applied. The morphological observation, performed with CLSM (confocal laser scanning microscopy) and with SEM (scanning electron microscopy), showed root canal dentin surface ablation and absence of debris in most of the irradiation conditions. Three-dimensional images revealed no smooth dentin walls. Strong melting and recrystallization, or unusually flat surfaces with open dentinal tubules, were obtained with sequences of three pulses without water cooling. This study demonstrated that varying the irradiation conditions affected the modifications of root canal surfaces [58] (Figs. 6.8, 6.9, 6.10, and 6.11).
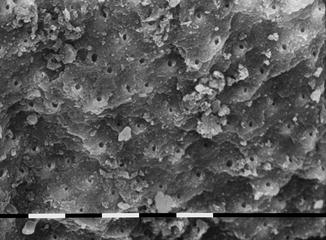
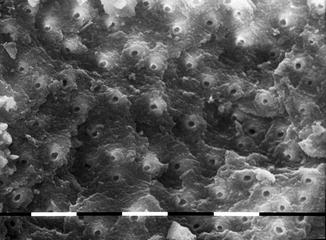
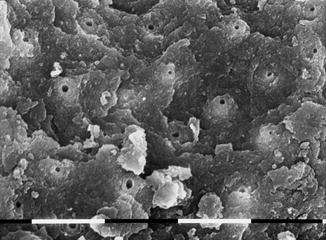
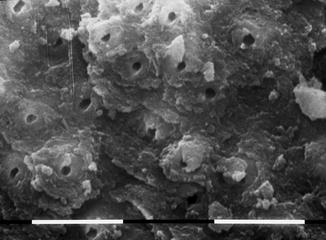

Fig. 6.8
SEM image of radicular dentin irradiated with Er,Cr:YSGG at 1.5 W, 20 Hz, air-water laser spray 35–25 %, 200 micron end-firing tip (2 mm/s speed). Signs of ablation, open dentinal tubules, and few debris are present

Fig. 6.9
SEM image of radicular dentin irradiated with Er,Cr:YSGG at 1.5 W, 20 Hz, air-water laser spray 35–25 %, 200 micron end-firing tip (2 mm/s speed). Typical signs of laser ablation, with typical chimneylike aspect, open dentinal tubules, and debris mainly absent in this area

Fig. 6.10
SEM image of radicular dentin irradiated with Er,Cr:YSGG at 1.5 W, 20 Hz, air-water laser spray 35–25 %, 200 micron end-firing tip (2 mm/s speed). Dentin surface shows superficial laser thermal damage. Dentin tubules are open with few debris

Fig. 6.11
SEM image of radicular dentin irradiated with Er,Cr:YSGG at 1.5 W, 20 Hz, air-water laser spray 35–25 %, 200 micron end-firing tip (2 mm/s speed). Dentin surface with open orifices and typical flaky surface related to laser-ablative thermal effect
6.4.3 Comparative Studies on Different Laser Wavelengths
A number of studies also compared the differing effects of several wavelengths on the canal surface. The real differences between wavelengths became clear, as well as the influence of some settings and modalities of use on the dentinal morphological changes after laser irradiation.
Takeda et al. (1998) compared the smear layer removal efficacy of three lasers: argon, Nd:YAG, and Er:YAG versus a control group irrigated with 17 % EDTA, after conventional instrumentation, in vitro. Statistical analysis showed significant differences between the control and the laser groups. Er:YAG laser irradiation (1 W, 100 mJ at 10 Hz) resulted in absence of smear layer and the presence of open dentinal tubules in the middle and apical one-third. The Nd:YAG laser (2 W, 200 mJ at 20 Hz) showed vaporization of the smear layer but also areas of fusion and recrystallization in the middle or apical one-third. The argon laser (1 W, 50 mJ at 5 Hz) showed the presence of pulp remnants in the middle one-third [27].
Kivanc et al. (2008), at the end of their comparative SEM studies on the effects of Nd:YAG and Er:YAG laser irradiation on radicular dentin, concluded that neither wavelength was efficient in removing the smear layer and debris from the canal walls [59].
Watanabe et al. (2010) evaluated thermal variations and dentin stress induced by laser irradiation, with or without air-water spray cooling. The canal was irradiated with Er:YAG and Nd:YAG lasers at a power of 1 W (100 mJ, 10 pps) per 5 s. The fiber was positioned 2 mm short from the apex. Using water cooling, the mean maximum strain induced by the Er:YAG laser was significantly lower than that of the Nd:YAG laser. However, without cooling, there were no significant differences between the two laser systems. The results suggested that the modifications induced by irradiation with water cooling were minimal and that the risk of microfractures remained in the case of insufficient cooling [31].
Esteves-Olveira et al. (2010) conducted a comparative study on morphological alterations and dentinal permeability variations after irradiation with three lasers of different wavelengths: 808 nm diode, Nd:YAG, and Er:YAG. Root-filled teeth were colored blue with 2 % methylene blue after irradiation, divided into two halves and photographed. The percentage of penetration of the colorant in the cervical, middle, and apical third was calculated by software for statistical analysis. The group irradiated with the Nd:YAG laser showed reduced penetration of colorant in all canal areas and melted surfaces and the presence of partially obliterated dentinal tubules. The diode and Er:YAG laser group showed more permeability, with values statistically superior to the control group and Nd:YAG. SEM analysis confirmed the variation of the observed canal permeability, showing canal surfaces with diffuse obliteration of dentinal tubules and areas of melting in the Nd:YAG group; the diode laser group showed surfaces with dentinal tubules mainly obliterated, while the Er:YAG group showed clean surfaces, without smear layer and with dentinal tubules open [60].
In investigations of the group of Ghent (Ghent Dental Laser Centre) by Michiels et al. (2010) and Vergauwen et al. (2014), the previously described effects of Nd:YAG and Er,Cr:YSGG on the root canal walls were confirmed [61, 62]. Michiels et al. (2010) demonstrated that the classic melting and glazing effects were absent root canals that were rinsed with EDTA (smear layer removed) [61]. When these effects occurred, these were seen in areas where the fiber had touched the root canal wall.
6.4.4 Laser and Irrigation for Root Canal Cleaning
Many studies reported that the combined action of both canal irrigation and laser irradiation led to better cleaned surfaces as compared to the action of just one solely. These studies, even if they were not always univocal, were an introduction to those of the laser activation of the irrigants (LAI) (see Chaps. 10 and 11).
The first lasers to be used in wet canals with water or irrigants were the Er:YAG laser [63–69] and the Er,Cr:YSGG laser [18, 19] and later the near-infrared laser [32, 43, 50, 52, 70].
Pecora et al. (2000) investigated in vitro the increase in dentinal permeability produced by the irradiation of Er:YAG lasers after endodontic instrumentation and irrigation with sodium hypochlorite. The study investigated different combinations of irrigation associated or not with laser irradiation, using deionized water or 1 % NaOCl. Erbium YAG lasers, used at 2.1 W (140 mJ, 15 Hz) for 20 s in distilled water, produced a major permeability increase. The use of laser with 1 % NaOCl after endodontic instrumentation, followed by a final rinsing with 1 % NaOCl, produced intermediate results, while irrigation alone, without the use of laser, resulted in the lowest permeability [63].
Brugnera et al. (2003) evaluated in vitro the effects of Er:YAG and Nd:YAG lasers on the permeability of canal dentin using distilled or deionized water or 1%NaOCl as irrigants after manual endodontic instrumentation. The results of the study were similar to those of Pecora et al. with Er:YAG laser irradiation with water producing higher dentin permeability. The use of 1 % NaOCl, with or without Er:YAG laser irradiation, showed intermediate results, while the use of 1 % NaClO and distilled or deionized water associated with the Nd:YAG laser showed a lower increment in permeability [64].
Biedma et al. (2005) compared the cleaning effects of EDTA and sodium hypochlorite irrigation alone or associated with Er:YAG laser irradiation at 1 W (100 mJ, 10 Hz), performed with a 285 micron fiber withdrawn with an apical-coronal movement at a speed of 2 m/s. The combination of irrigation-laser irradiation produced better cleaning. However, there were areas not perfectly cleaned with obliterated dentinal tubules [65].
Similar results were obtained by Altusandar et al. (2006) using the Er,Cr:YSGG laser, sodium hypochlorite, and a urea peroxide gel (RC-Prep). SEM observation revealed insufficient removal of the smear layer from the group irrigated solely with 5.25 % NaOCl; the group treated with a combination of RC-Prep and 5.25 % NaOCl showed a moderate presence of smear layer but also clear areas of exposed collagen. The group treated with a combination of 5.25 % NaOCl irrigation and Er,Cr:YSGG laser irradiated, followed again by NaOCl irrigation, showed the major removal of the smear layer, even if some areas of thermal damage, with carbonization and melting were observed [66].
Radatti et al. (2006) compared the efficacy of the Er,Cr:YSGG laser associated with two irrigation protocols and conventional irrigation protocols alone for the removal of debris after endodontic instrumentation, using 0.5 ml distilled water or 5.25 % NaOCl. Laser irradiation with just distilled water leaves more smear layer compared with conventional irrigation. Laser irradiation with 5.25 % NaOCl did not result in statistically significant differences with conventional irrigation [67].
Varella et al. (2007) evaluated numbers of lateral canals and isthmus obturated after different treatments: 17% EDTA for 3 minutes, Er,Cr:YSGG laser for 40 s, and control group. The laser-irradiated group showed a statistically superior number of obturated canals/isthmus [68].
Watanabe et al. (2010) evaluated thermal variations or dentinal stress induced by laser irradiation with water cooling and concluded that these were minimal and that the risk of microfractures remained in case of insufficient cooling [31].
Kalyoncuoğlu and Demiryürek (2013) analyzed with SEM the canal surface of mono-radicular teeth after chemomechanical preparation followed by different methods of final irrigation: 5.25 % NaOCl, 17 % EDTA, BioPure MTDA, Er:YAG laser irradiation at 1.8 W (120 mJ, 15 Hz), or irradiation with Nd:YAG laser at 1 W (100 mJ, 15 Hz). Irrigation with EDTA and NaOCl brought about superior cleaning in every canal area, without statistically relevant differences. All the techniques produced an efficient cleaning action [69].
In the near-infrared spectrum of light, both the 810 nm laser, the 940 and 980 nm lasers, as well as the 1064 Nd:YAG laser were studied in association with different irrigants as the final step, for cleaning and decontamination at the end of endodontic treatment. Several studies (Santos et al. 2005; Gurbuz et al. 2008; Faria et al. 2008; Marchesan et al. 20081; Alfredo et al. 2009 and Olivi et al. 2013) reported that when the canal is irradiated after or together with different irrigants (distilled water, EDTAC, chlorhexidine, sodium hypochlorite), the morphological pattern is better or similar to that produced by just the irrigation alone [32, 43, 50, 52, 70, 71] (Figs. 6.12, 6.13, 6.14, 6.15, 6.16, and 6.17).
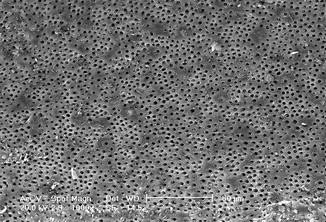

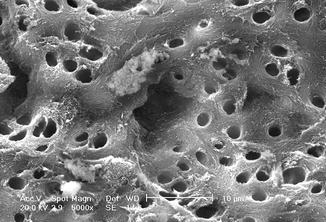
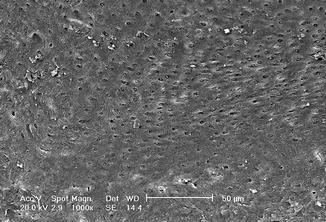
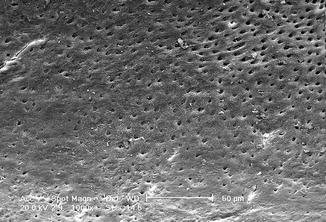

Fig. 6.12
SEM image (1000×) of radicular dentin irradiated with 810 nm diode laser at 2.5 W in gated mode (20 ms ton and 20 ms toff) with 200 micron tip, in root canal irrigated with 17 % EDTA (2 mm/s speed). Dentin surface with dentin tubules mainly opens with few debris and smear layer (Reprinted with permission from Olivi et al. [70] (Fig. 3))

Fig. 6.13
SEM image (5000×) of radicular dentin irradiated with 810 nm diode laser at 2.5 W in gated mode (20 ms ton and 20 ms toff) with 200 micron tip, in root canal irrigated with 17 % EDTA (2 mm/s speed). Dentin surface with dentin tubules mainly opens with some debris and smear layer. The EDTA action produces partial exposure of intertubular and peritubular collagenic fibers. No thermal damage visible (Reprinted with permission from Olivi et al. [70] (Fig. 4))

Fig. 6.14
SEM image (5000×) of radicular dentin irradiated with 810 nm diode laser at 2.5 W in gated mode (20 ms on and 20 ms off) with 200 micron tip, in root canal irrigated with 17 % EDTA (2 mm/s speed). Dentin surface with dentin tubules mainly opens with some debris and smear layer. The EDTA action produces partial exposure of intertubular and peritubular collagenic fibers. No thermal damage visible (Reprinted with permission from Olivi et al. [70] (Fig. 5))

Fig. 6.15
SEM image (1000×) of radicular dentin irradiated with 810 nm diode laser at 2.5 W in gated mode (20 ms on and 20 ms off) with 200 micron tip, in root canal irrigated with 5 % NaOCl (2 mm/s speed). Dentin surface is quite clean, but orifices of dentin tubules are mainly plugged from debris and dentin tubules. Laser thermal damage is not visible (Reprinted with permission from Olivi et al. [70] (Fig. 6))

Fig. 6.16




SEM image (1000×) of radicular dentin irradiated with 810 nm diode laser at 2.5 W in gated mode (20 ms on and 20 ms off) with 200 micron tip, in root canal irrigated with 5 % NaOCl (2 mm/s speed). Dentin surface is quite clean, but orifices of dentin tubules are mainly plugged from debris and dentin tubules. Laser thermal damage is not visible (Reprinted with permission from Olivi et al. [70] (Fig. 7))
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


