The comprehensive treatment of cleft lip and palate deformities requires thoughtful consideration of the anatomic complexities of the deformity and the delicate balance between intervention and growth. Comprehensive and coordinated care from infancy through adolescence is essential to achieve the best outcome, and surgeons with formal training and experience in all the phases of care must be actively involved in the planning and treatment. Specific goals of surgical care for children born with cleft lip and palate include the following:
- •
Normalized esthetic appearance of the lip and nose
- •
Intact primary and secondary palate
- •
Normal speech, language, and hearing
- •
Nasal airway patency
- •
Class I occlusion with normal masticatory function
- •
Good dental and periodontal health
- •
Normal psychosocial development
Successful management of the child born with a cleft lip and palate requires coordinated care provided by a number of different specialties, including oral and maxillofacial surgery, otolaryngology, plastic surgery, genetics and dysmorphology, speech-language pathology, orthodontics, pediatric dentistry, and prosthodontics. In most cases care of patients with congenital clefts has become a subspecialty area of clinical practice within these different professions. In addition to the primary cleft repairs in infancy, treatment plans routinely involve multiple treatment interventions to achieve the above goals staged throughout childhood. Because care is provided over the entire course of the child’s development, long-term follow-up is essential under the care of these different health care providers.
The formation of interdisciplinary cleft palate teams has served two key objectives of successful cleft care: (1) coordinated care provided by all the necessary disciplines, and (2) continuity of care with close-interval follow-up of the patient throughout periods of active growth and ongoing stages of reconstruction. The best outcomes are achieved when the team’s care is centered on the patient, family, and community rather than a particular surgeon, specialty, or hospital. The idea of having an objective team that does not revolve around the desires of one particular individual or discipline is sometimes impeded by competitive interactions between surgical specialties. Historic battles over surgical domains between surgical specialties and economic factors contribute to these conflicts and negatively affect the work of the team. Healthy team dynamics and optimal patient care are achieved when all members are active participants, when team protocols and referral patterns are equitable, and when the needs of the child are placed above the needs of the team or individual members. Treatment should be based on the surgeon’s formal training and experience instead of specialty identity.
The surgical reconstruction of clefts requires that the surgeon undertaking this important work maintain a cognitive understanding of the complex malformation itself, the varied operative techniques used, facial growth considerations, and the psychosocial health of the patient and family. This chapter presents the overall staged reconstructive approach for repair of cleft lip and palate from infancy through the time of skeletal maturity as well as a discussion of the surgical procedures involved in primary cleft lip and palate repair. Controversies in cleft repair, secondary revision procedures, bone graft reconstruction of the cleft maxilla, and orthognathic surgery for cleft-related dysmorphology are discussed in later chapters of this text.
HISTORY OF CLEFT LIP AND PALATE REPAIR
The history of cleft lip and palate care has always been closely linked to dentistry and oral and maxillofacial surgery. The birth of what is now the American Cleft Palate-Craniofacial Association is strongly rooted in dentistry, and its further development has always had various aspects of the dental profession at its heart.
The first documented cleft lip repair was performed in 390 ad on a patient who later became the governor general of several regions in China, although nothing is known about the actual surgeon. Jehan Yperman is believed to have been the first to describe unilateral and bilateral cleft lip repair. The first diagrammatic representation of cleft lip repair and cleft palate obturator use is credited to Ambrose Pare in the fourteenth century. Much later, the first documented successful cleft palate repair was performed by a dentist, Le Monnier, in 1766 in Paris. The concepts of cleft lip and palate repair have evolved from straight-line repairs to a variety of techniques using various cutbacks, triangles, and Z-plasties. During the 1950s Asensio, an oral and maxillofacial surgeon from Guatemala, developed a novel technique for cleft lip repair that involved the rotation of the philtral segment inferiorly and advancement of the lateral segment medially with a quadrangular flap. Although he used this approach in Guatemala throughout the 1950s, he did not report it until much later. Ralph Millard described his classic rotational advancement technique in the mid-1950s, and his concepts changed cleft repair forever. Millard is credited with perhaps the most important technical development related to cleft lip repair, and today the majority of surgeons use his original technique or some close modification of it.
In the mid-nineteenth century, Hullihen, recognized as the father of American oral and maxillofacial surgery, published a treatise on comprehensive care of cleft lip and palate deformities. Another pioneer, Truman Brophy, was the professor of oral surgery and dean of the Chicago College of Dentistry and contributed greatly to the care of many patients with clefts. Brophy published a text detailing his experiences with the management of various malformations of the mouth and their surgical repairs, including the details of cleft repair. One of his pupils was Chalmers Lyons, who started a residency program in oral surgery at the University of Michigan in 1917. Lyons developed the largest cleft practice in America and contributed extensively to the literature.
Many of the concepts related to interdisciplinary care with a cleft plate team were introduced by Robert Ivy, an oral and maxillofacial surgeon who later became dually qualified in plastic surgery. Ivy trained in both dentistry and medicine at the University of Pennsylvania. After his training in dentistry, Ivy further developed his interests in maxillofacial surgery as an assistant to his uncle, Matthew Cryer, who was a professor in oral surgery at the University of Pennsylvania. Robert Ivy became interested in clefts during his training as the first dental intern at Philadelphia General Hospital at the University of Pennsylvania. His interests in maxillofacial injury led him to serve in France in World War I as an assistant to Vilray Blair. After the war, Ivy and Blair’s collaboration resulted in two landmark publications by Ivy, Essentials of Oral Surgery and Fractures of the Jaws . Through work with his state representatives in Harrisburg, Penn., he was able to start the first cleft palate clinics in Lancaster, Pittsburgh, Philadelphia, Erie, and Scranton, which provided interdisciplinary care to children in those regions for cleft lip and palate deformities. When Reed Dingman put forth a resolution of the American Society of Maxillofacial Surgeons condemning oral and maxillofacial surgeons practicing in the hospital setting, Ivy resigned his membership and sent a letter of protest in support of his dental colleagues to the organization that he helped build.
In the 1950s the concept of primary or early bone grafting of the cleft maxillary defect was introduced by Schmid. Although the concept initially was met with enthusiasm from a number of surgeons, primary bone grafting eventually was abandoned because of unfavorable outcomes. During the decades that followed, the negative skeletal, dental, and growth-related consequences of primary bone grafting became better understood. During the early 1970s an oral and maxillofacial surgeon, Phillip Boyne, was the first to publish his favorable outcomes using autogenous particulate bone grafts for reconstruction of the cleft maxilla/alveolus later in childhood during the mixed dentition rather than earlier in life. Although Boyne’s work and results represented a landmark discovery in the field of cleft reconstruction, cleft palate teams were slow to integrate his approach into their treatment protocols because of the negative associations that lingered after the days of primary bone grafting. Today Boyne’s principles of secondary bone grafting represent the standard approach for most of the world’s cleft centers.
Orthognathic reconstruction of the patient with cleft deformities has been discussed by many authors. Early techniques limited some surgeons’ options to procedures centered on mandibular setback. During the 1970s the use of total maxillary osteotomy was pioneered by Bell. His novel ideas provided oral and maxillofacial surgeons with an understanding of the biologic basis for maxillary osteotomy, described the vascular supply that allowed the procedures to be safely performed and, as a result, incorporated the Le Fort I osteotomy into modern practice. Since that time a number of technical refinements have been described for use of the Le Fort I osteotomy specifically in the cleft patient. Much of this work has been done by two of Bell’s former pupils, Fonseca and Turvey, who went on to make substantial contributions to the skeletal reconstruction of patients with clefts. Another dually qualified oral and maxillofacial surgeon, Posnick, has published the most complete descriptions of surgical technique modifications for patients undergoing midfacial advancement in the absence of prior bone graft reconstruction and his extensive experiences with the long-term stability of midfacial advancement after correction of various types of cleft deformities with orthognathic techniques. Distraction osteogenesis has gained recent popularity for correction of midfacial hypoplasia but has yet to show significant advantages over traditional techniques for the majority of patients.
Comprehensive and coordinated care has become more prevalent across the world, involving many different types of specialty care for children with clefts. Evidenced-based care is becoming more of a reality, with pooled data and continued research worldwide. The days of performing procedures purely based on a particular surgeon’s clinical experience are passing. Posnick, Shaw, and others have provided comprehensive, succinct, and evidenced-based discussions on the topics of cleft lip and palate reconstruction from infancy through adolescence that are beginning to take hold in the form of treatment protocols. Dentistry and the specialty of oral and maxillofacial surgery have had significant roles in analyzing data and putting forth new standards of care. These efforts as well as craniofacial training programs associated with oral and maxillofacial surgery departments have helped solidify the role of oral and maxillofacial surgery in the comprehensive care of patients with clefts.
EMBRYOLOGY
To understand the goals of lip and palate repair from an anatomic standpoint, the cleft surgeon must have an appreciation for the failure of embryogenesis that results in clefting. Critical points in the development of the fetus occur when the fusion of various prominences creates continuity and form to the lip, nose, and palate. Anomalies occur when the normal developmental process is disturbed between these components. Each prominence is composed of ectomesenchyme derived from neural crest tissue of the mesencephalon and rhombencephalon. Mesoderm also is present within these prominences as mesenchymal tissue. The prescribed destiny of each of these cells and tissues is controlled by various genes to alter the migration, development, and apoptosis and form the normal facial tissues of the fetus. At the molecular level many interdependent factors exist, such as signal transduction, mechanical stress, and growth factor production, that affect the development of these tissues. Currently only portions of this complex interplay of growth, development, and apoptosis are clear.
At approximately 6 weeks of human embryologic development the median nasal prominence fuses with the lateral nasal prominences and maxillary prominences to form the base of the nose, nostrils, and upper lip. The confluence of these anterior components becomes the primary palate. When this mechanism fails, clefts of the lips and/or maxilla occur. At approximately 8 weeks’ gestation the palatal shelves elevate and fuse with the septum to form the intact secondary palate. When one palatal shelf fails to fuse with the other components, a unilateral cleft of the secondary palate occurs. If both palatal shelves fail to fuse with each other and the midline septum, a bilateral cleft of the palate occurs.
Fusion occurs when programmed cell death (apoptosis) occurs at the edges of the palatal shelves. The ectodermal component disintegrates and the mesenchyme fuses to form the intact palate. Soon after this the anterior primary palate fuses with the secondary palate and ossification occurs. If failure of fusion occurs with any of the above components at any point, a cleft of the primary and/or secondary palates occurs. Clefts may be complete or incomplete based on the degree of this failure of fusion.
EMBRYOLOGY
To understand the goals of lip and palate repair from an anatomic standpoint, the cleft surgeon must have an appreciation for the failure of embryogenesis that results in clefting. Critical points in the development of the fetus occur when the fusion of various prominences creates continuity and form to the lip, nose, and palate. Anomalies occur when the normal developmental process is disturbed between these components. Each prominence is composed of ectomesenchyme derived from neural crest tissue of the mesencephalon and rhombencephalon. Mesoderm also is present within these prominences as mesenchymal tissue. The prescribed destiny of each of these cells and tissues is controlled by various genes to alter the migration, development, and apoptosis and form the normal facial tissues of the fetus. At the molecular level many interdependent factors exist, such as signal transduction, mechanical stress, and growth factor production, that affect the development of these tissues. Currently only portions of this complex interplay of growth, development, and apoptosis are clear.
At approximately 6 weeks of human embryologic development the median nasal prominence fuses with the lateral nasal prominences and maxillary prominences to form the base of the nose, nostrils, and upper lip. The confluence of these anterior components becomes the primary palate. When this mechanism fails, clefts of the lips and/or maxilla occur. At approximately 8 weeks’ gestation the palatal shelves elevate and fuse with the septum to form the intact secondary palate. When one palatal shelf fails to fuse with the other components, a unilateral cleft of the secondary palate occurs. If both palatal shelves fail to fuse with each other and the midline septum, a bilateral cleft of the palate occurs.
Fusion occurs when programmed cell death (apoptosis) occurs at the edges of the palatal shelves. The ectodermal component disintegrates and the mesenchyme fuses to form the intact palate. Soon after this the anterior primary palate fuses with the secondary palate and ossification occurs. If failure of fusion occurs with any of the above components at any point, a cleft of the primary and/or secondary palates occurs. Clefts may be complete or incomplete based on the degree of this failure of fusion.
GENETICS AND ETIOLOGY
Clefts of the upper lip and palate are the most common major congenital craniofacial abnormality and are present in approximately 1 in 700 live births. Although inheritance may play a role, cleft lip and palate is not considered a single-gene disease. Instead, clefts are thought to be of a multifactorial etiology with a number of potential contributing factors. These factors may include chemical exposures, radiation, maternal hypoxia, teratogenic drugs, nutritional deficiencies, physical obstruction, and genetic influences. One prevailing theory relates the process of clefting as a point when multiple factors come together to raise the individual above a threshold, at which time the mechanism of fusion fails. Multiple genes recently have been implicated in the etiology of clefting. Some of these genes include the MSX , LHX , goosecoid , and DLX genes. Additional disturbances in growth factors or their receptors that may be involved in the failure of fusion include fibroblast growth factor, transforming growth factor, platelet-derived growth factor, and epidermal growth factor.
Clefts of the lip occur more commonly in males than in females. In addition, left-sided cleft lips are more common than right-sided cleft lips, and unilateral cleft lips are more common than bilateral cleft lips. Bilateral clefts of the lip are most often associated with clefting of both the primary and secondary palates. Cleft palate alone is seen in approximately 1 in 2000 live births, an incidence similar in all racial groups. Significant differences in the prevalence of clefts exist when specific ethnic and racial populations are examined. For example, African Americans have a birth prevalence that is less common than the total population, and Asians tend to have a higher prevalence.
In the majority of cases unilateral cleft lip and palate is an isolated nonsyndromic birth defect not associated with any other major anomalies. By comparison, a much greater proportion of patients with an isolated cleft palate have an associated syndrome or sequence. Some of the more common syndromes seen in this group include Stickler, van der Woude, and DiGeorge syndromes. Early diagnosis is important because functional issues may arise early in life and go unnoticed. For example, patients with an isolated cleft palate should be evaluated early by an experienced pediatric ophthalmologist to evaluate the possibility of Stickler syndrome. Patients with Stickler syndrome may have ocular abnormalities that lead to retinal detachment. In an otherwise healthy-appearing child these findings may be difficult to diagnose, so early visual loss may go unnoticed. In many cases long-term genetic follow-up is necessary to make a definitive diagnosis and provide genetic counseling.
The chances of a recurrence of clefting within a family depend on many factors, including family history, severity, gender, degree of relationship to the affected individual, and the expression of a syndrome. Predicting the inheritance patterns of families with a history of cleft lip and/or palate can be complicated. A skilled geneticist or dysmorphologist is best equipped to make these determinations based on pedigree analysis and genetic testing. Because most clefts are sporadic, the chances of a family having another child with a cleft after having a child with a unilateral cleft lip and palate in which no family history of clefting is present is approximately 2% to 4%. The chances are higher if additional family history is present or if the cleft is bilateral. The nature of any genetic influence will have an effect on the presence of a cleft. Such is the case in patients with autosomal-dominant syndromes such as Stickler syndrome, in which 50% of the children may express the syndrome if one of the parents carries the altered gene.
CLASSIFICATION
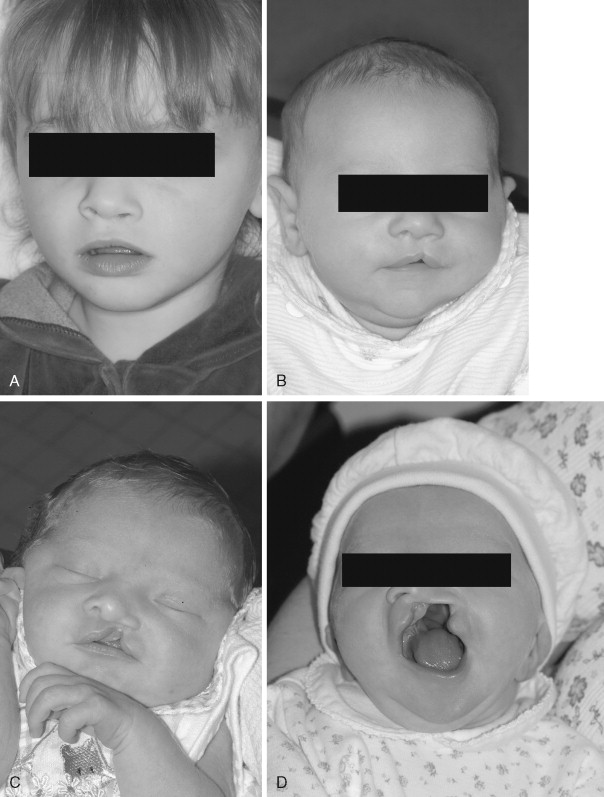
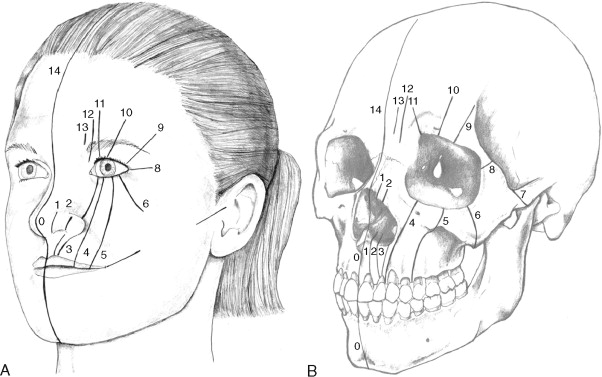
The typical classification system used clinically to describe standard clefts of the lip and palate is based on careful anatomic description. Clefts can be unilateral or bilateral; microform, incomplete, or complete; and may involve the lip, nose, primary palate, and/or secondary palates ( Figure 35-1 ). The presentation of clefts is extremely variable, and the individual repairs are custom tailored to achieve the best symmetry and balance. More severe facial clefting is most commonly described by using Paul Tessier’s orbitocentric system of numbering ( Figure 35-2 ). Other systems are based on embryologic fusion planes, but are these are cumbersome to use in routine clinical practice.
PRENATAL COUNSELING
Recent advances in imaging have revolutionized prenatal care and maternal-fetal medicine. Ultrasound images of clefts of the lip currently can be visualized at approximately 16 weeks of gestation. Diagnostic images of the palate are more difficult to acquire, making the correct prenatal diagnosis of a cleft palate less predictable. Anterior palatal structures may be visualized by using sagittal and coronal views, but this currently requires the latest technology and a skilled ultrasonographer with experience performing this type of study.
When the diagnosis of cleft lip is made during pregnancy, the family can be referred to an experienced surgeon for a prenatal discussion. A prenatal consultation provides an excellent opportunity to explain the diagnosis, review the different stages of cleft lip and palate reconstruction that may be necessary, and prepare the parents for practical considerations such as feeding a child with a cleft palate. This gives the family the opportunity to ask questions, calm fears, and learn about feeding techniques that will be important during the first week of life for their baby. Parents are empowered with this new knowledge, and the preparations made during a prenatal consultation allow them to anticipate the delivery of their baby with a greater comfort level regarding the necessary care of the infant during the early postnatal period. The family is then referred to a cleft and craniofacial team to begin a more thorough interdisciplinary approach.
Critical to this process is consultation with a geneticist or dysmorphologist to discuss the issues associated with the birth and the possibility of other associated deformities. Additional testing may be warranted to evaluate the possibility of associated deformities, syndromes, or sequences that could affect the birthing process. Exceptionally skilled ultrasonographers can visualize airway development and other abnormalities that may require early intervention with fetal surgery, exit procedures, extracorporeal membrane oxygenation, or surgical airway management (tracheotomy) at the time of delivery. Magnetic resonance imaging often is helpful in determining the more definitive morphology and helping guide the need for intervention early in life.
In some medical centers fetal diagnosis and treatment teams are in place to deal with issues associated with various deformities diagnosed in the prenatal period. These teams foster a cohesive environment in which information is exchanged through consultation. Much like the environment of a cleft and craniofacial team, families can get the best information available to consider their baby’s treatment decisions with an interdisciplinary care model that is patient (mother and fetus), family, and community oriented.
FEEDING THE CHILD WITH A CLEFT PALATE
Children born with isolated cleft lip can feed quite well and even have the opportunity to breastfeed in most instances. However, infants with cleft palate can have difficulty feeding because of the inability to form an adequate seal between the tongue and palate to create sufficient negative pressure to suck fluid from a bottle. Nasal regurgitation and inefficient handling of secretions and foods also may be observed during early development. Specialized nipples and bottles are necessary to improve feeding immediately after birth. The most useful devices combine oversized nipples with reservoir spaces and large openings, a squeezable bottle to push fluid into the nipple assembly, and a one-way valve that allows the bolus of fluid to pass from the bottle to the nipple only to minimize the amount of work the child must perform to feed. These include a variety of nipples with reservoirs that collect a variable volume of liquid that can be expressed more easily when sucking is inefficient or not possible. Bottles that can be squeezed to allow manual flow of liquid to the infant are helpful for improving feeding. No single bottle and nipple combination works better than another; trials with a variety of types using different techniques are helpful to optimize feeding early in life.
Close attention to weight gain is necessary for these children. In general, in 24 hours each infant should ingest approximately 2 to 3 oz of milk for each pound of weight. Feeding sessions should last no longer than 35 minutes because longer sessions are fatiguing and burn more calories than the baby can consume. Infants should be weighed at least weekly on the same scale, preferably at the pediatrician’s office.
The subject of breastfeeding an infant with a cleft palate is controversial, with some practitioners encouraging the practice and others strongly opposed to it. Breastfeeding a newborn has clear advantages, including passive immunologic contribution of the mother to the child in the form of secretory immunoglobulin A and an experience that enhances bonding between the mother and child during such a critical period. At the same time the infant’s inability to create negative oral pressure often makes successful nursing difficult, if not impossible. Exclusively breast-fed infants with severe dehydration and failure to thrive are relatively common because of these difficulties. This is especially a concern in infants with a wide cleft of the secondary palate, a condition in which breast feeding may not be possible. The authors’ approach with regard to breastfeeding in the presence of a cleft palate is to use a combined protocol that includes intermittent feeding with the use of a specialized bottle (as described above) and attempts at nursing. Breast milk may be pumped for use with the specialized nipple and bottle that will provide the nutritional and immunologic benefits desired. This also allows the parents to keep a more quantitative record of how many ounces have been ingested over the course of the day; this normally is difficult with breastfeeding alone. At the same time, the mother and baby are not deprived of an opportunity to incorporate breastfeeding into the daily regimen. This approach obviously requires rigorous documentation of the child’s weight, consultation with a lactation consultant and infant feeding specialist, and frequent follow-up evaluations through the surgeon and/or pediatrician.
TREATMENT PLANNING AND TIMING
The timing of cleft lip and palate repair is controversial. Despite a number of meaningful advancements in the care of patients with cleft lip and palate, a lack of consensus exists regarding the timing and specific techniques used during each stage of cleft reconstruction. Surgeons must continue to balance the functional needs, esthetic concerns, and the issue of ongoing growth carefully when deciding how and when to intervene. In no other type of surgical problem is the issue of early surgery’s effect on growth more apparent than in the treatment of cleft lip and palate deformities. The decision to manipulate the tissues of the growing child surgically should not be made lightly and should consider the possible growth restriction that can occur with early surgery. Nevertheless, many patients with congenital deformities benefit from surgical intervention based on functional or psychosocial reasons. Understanding the growth and development of the craniofacial skeleton is critical to the treatment planning process. In many cases waiting for a greater degree of growth to occur is advantageous unless compelling functional or esthetic issues are present that cannot or should not wait.
Because of the many different treatment philosophies, the timing of treatment interventions varies considerably among cleft centers. Therefore producing a timing regimen that everyone agrees on is difficult. Each stage of surgical reconstruction and the suggested timing based on the patient’s age are presented in Table 35-1 . Special considerations may alter the sequencing or timing of the various procedures based on individual functional or esthetic needs. Significant differences exist worldwide regarding the timing of different repairs. As of yet, timing of repair cannot be guided by truly definitive outcome research.
| Procedure | Time Frame |
|---|---|
| Cleft lip repair | After 10 weeks |
| Cleft palate repair | Age 9-18 months |
| Pharyngeal flap or pharyngoplasty | Age 3-5 years or later based on speech development |
| Maxillary/alveolar reconstruction with bone grafting | Age 6-9 years based on dental development |
| Cleft orthognathic surgery | Age 14-16 years in girls, 16-18 years in boys |
| Cleft rhinoplasty | After age 5 years, but preferably at skeletal maturity; after orthognathic surgery when possible. |
| Cleft lip revision | Anytime once initial remodeling and scar maturation is complete, but best performed after age 5 years |
Cleft lip repair generally is undertaken at some point after 10 weeks of age. Waiting until the child is 10 to 12 weeks of age is allows enough time for a complete medical evaluation of the patient so that any associated congenital defects affecting other organ systems (e.g., cardiac or renal anomalies) may be uncovered. The surgical procedure itself may be easier when the child is slightly larger and the anatomic landmarks are more prominent and well defined. Historically, the anesthetic risk-related data have suggested that the safest time for surgery in this population of infants could be outlined simply by using the “rule of 10’s.” This referred to the idea of delaying lip repair until the child was at least 10 weeks old, 10 pounds in weight, and with a minimum hemoglobin level of 10 mg/dL. Today more sophisticated pediatric anesthetic techniques, advances in intraoperative monitoring, and improved anesthetic agents have all resulted in the ability to provide safe general anesthesia much earlier in life. Despite this ability, no measurable benefit exists to performing lip repair before 3 months of age. Some surgeons have advocated that lip repair be carried out in the first days of infancy based on the idea of capitalizing on early “fetal-like” healing. Unfortunately, these hoped-for benefits have not been observed, and problems with excessive scarring and less-favorable outcomes have been encountered. Children may have more scarring at this early age, and their tissues are smaller and more difficult to manipulate. Consequently the esthetic outcomes may be worse if surgery is performed at an earlier age; because no clear benefits exist for earlier repair, the recommendations for repair remain at approximately 3 months of age.
Cleft palate repair usually is performed at approximately 9 to 18 months of age. In deciding the timing of repair the surgeon must consider the delicate balance between facial growth restriction after early surgery and speech development that requires an intact palate. Most children require an intact palate to produce certain speech sounds by 18 months of age. If developmental delay is present and speech will not likely develop until later, then the repair can be delayed further. Little evidence suggests any benefit to palate repair before 9 months of age. Repairs before this time are associated with a much higher incidence of maxillary hypoplasia later in life and show no improvements in speech. For these reasons most surgeons perform primary palate repair at approximately 9 to 18 months of age.
As these children continue to develop, a significant percentage will have inadequate closure of the velopharyngeal mechanism (velopharyngeal insufficiency [VPI]) even after successful palate repair, which may produce hypernasal speech. These children usually are diagnosed at 3 to 5 years of age when a detailed speech examination can be obtained by a skilled speech pathologist. When VPI is shown to be consistent and caused by a definable anatomic defect, surgery often is helpful. A pharyngeal flap or sphincter pharyngoplasty may be used to treat VPI, with the goal of improving closure between the oral and nasal cavities and reducing nasal air escape during the production of certain sounds. In some select cases, palate repeat repair may be considered with a two-flap palatoplasty or double-opposing Z-plasty technique.
Approximately 75% of patients with any type of cleft have clefting of the maxilla and alveolus. Bone graft reconstruction of this site is performed during the mixed dentition before the eruption of the permanent canine and/or the permanent lateral incisor. The timing of this procedure is based on dental development and not chronologic age. On the basis of work by Boyne, most surgeons reconstruct this area during the mixed dentition before eruption of the permanent canine. Earlier reconstruction of this area has been associated with a high degree of maxillary growth restriction, requiring orthognathic correction later in life in a much higher percentage of patients. The gold standard for reconstruction in this area is autogenous bone from the anterior iliac crest. Cranial bone, rib, tibia, symphysis of the mandible, zygoma, and allogeneic bone have all been studied, but none has been shown to be appreciably better than the iliac crest.
Orthognathic reconstruction of maxillary and mandibular discrepancies is performed at 14 to 18 years of age based on individual growth characteristics. This is done in conjunction with orthodontics before and after surgery. However, in some cases of severe maxillary hypoplasia, early Le Fort I osteotomy may be performed to optimize facial esthetics and occlusion with the supposition that revision osteotomies will likely be necessary. These early osteotomies may complicate later treatment. Early orthognathic maxillary advancement is reserved for the most severe dysmorphology, and in most cases the authors prefer standard orthognathic techniques timed in the standard fashion. Attempts at using distraction osteogenesis have been associated with a higher complication rate than standard orthognathic techniques.
As with the timing of other interventions, lip and nasal revision is best reserved for after the majority of growth is complete. Most of the lip and nasal growth is complete after age 5 years. Lip revision may be considered just before school begins, at approximately 5 years of age or later. However, this may be performed earlier if the deformity is severe. Nasal revision is performed after age 5 years because most of the nasal growth is complete by this time. If orthognathic reconstruction is likely, then rhinoplasty usually is best performed after orthognathic surgery because maxillary advancement improves many characteristics of nasal support. However, when nasal deformity is particularly severe, rhinoplasty can be considered earlier even if orthognathic surgery is expected. Multiple early revisions of the lip or nose should be avoided so that excess scarring does not potentially impair ongoing growth.
CLEFT LIP AND PALATE REPAIR
PRESURGICAL TAPING AND PRESURGICAL ORTHOPEDICS
Facial taping with elastic devices may be used for application of selective external pressure and may improve lip and nasal position before the lip repair procedure. In the authors’ opinions these techniques often have greater impact in cases of wide bilateral cleft lip and palate in which manipulation of the premaxillary segment may make primary repair technically easier. Although one of the basic surgical tenets of wound repair is to close wounds under minimal tension, attempts at improving the arrangement of the segments by using taping methods have not shown a measurable improvement.
Some surgeons prefer presurgical orthopedic (PSO) appliances rather than lip taping to achieve similar goals. PSO appliances are composed of a custom-made acrylic base plate that provides improved anchorage in the molding of lip, nasal, and alveolar structures during the presurgical phase of treatment ( Figure 35-3 ). Although the use of appliances probably allows an easier surgical repair, clinical evidence is lacking to demonstrate any measurable improvement in esthetics of the nose or lip, dental arch relation, tooth survival, or occlusion of the patient. Studies have looked at the dental arch relations and other outcomes in patients who have infant presurgical orthopedic devices, and no improvement was seen. In addition, no long-term improvement in speech outcome has be demonstrated in patients who had PSOs. Furthermore, concerns regarding potential negative consequences of these types of appliances have been raised. PSOs also add significant cost and time to treatment early in the child’s life. Many appliances require a general anesthetic for the initial impression used to fabricate the device. Frequent appointments are necessary to monitor the anatomic changes and periodic appliance adjustment.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


