Fig. 11.1
OLP lesions affecting tongue and oral mucosa superimposed by oral candidosis
The objective of this chapter is to review the current literature on the association of candidosis and oral lichen planus, providing important information for a correct diagnosis and adequate treatment of these associated diseases.
Oral Lichen Planus (OLP)
Lichen planus is a relatively common chronic inflammatory disease with a prevalence of oral manifestations between 0.1 and 2.2 % of the population. First described in 1869 by Erasmus Wilson, the disease may involve skin and mucosa, individually or simultaneously. Lesions can be restricted to the mouth or with cutaneous involvement (Navas-Alfaro et al. 2003).
The aetiology of lichen planus has not been completely elucidated; however, it is considered a multifactorial disease mediated by an immunopathological mechanism, particularly involving T lymphocytes (Dorta et al. 2000). In a significant number of cases, the disease is associated with anxiety and depression, according to García-Pola Vallejo et al. (2001). It occurs in patients of both sexes, with a predominance in women, generally over 40 years old (Souza and Rosa 2008).
A total of 50 dermatologists from Bauru/SP/Brazil completed a questionnaire of 19 questions about aetiology, diagnosis and treatment of lichen planus. The main etiological factors reported included idiopathic (22.4 %), psychosomatic (21.5 %), immune (14.0 %) and viral (14.0 %) factors. Other factors related to the disease pathogenesis mentioned by the clinicians were diabetes mellitus, infections, anaemia, genetic predisposition, allergies, smoking habits and sun exposure (Dorta et al. 2000).
According to Souza and Rosa (2008), stress; food such as tomatoes, citrus fruits and spicy dishes; dental procedures; systemic diseases; abuse of alcohol and use of any source of tobacco are associated to periods of exacerbation of the disease. The concern of its association with systemic diseases, especially hepatitis C virus (HCV) infections, is rising in recent years.
The OLP is a disease of polymorphous clinical appearance, being classified as typical or atypical form. The typical form can be divided into reticular (Wickham striae) (Fig. 11.2), popular, plaque and warty. Atypical forms are ulcerated, atrophic, erythematous, erosive (Fig. 11.3) and bullous pigment. In the mouth, it occurs mainly at the buccal mucosa, bilaterally, at the margin and dorsum of the tongue and at the lip (Souza and Rosa 2008).
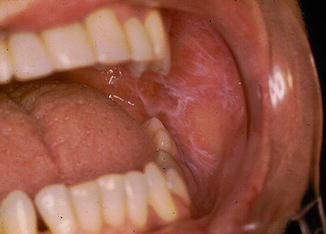
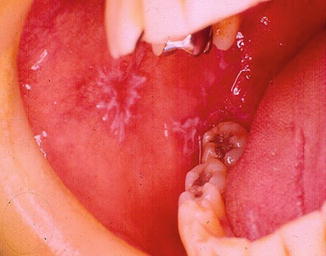

Fig. 11.2
Reticular OLP at the left oral mucosa

Fig. 11.3
Erosive OLP at the right oral mucosa
Essentially, there are two forms of oral lesions: reticular and erosive. The reticular lichen planus is much more common than the erosive form. However, several studies report the erosive form predominantly, because it is symptomatic and, in many cases, is the only treated variety of OLP. The reticular form usually presents no symptoms and involves the posterior region of the buccal mucosa bilaterally. Other areas of the oral mucosa are likely to be affected, such as the lateral border and dorsum of the tongue, gums, palate and lip vermilion (Navas-Alfaro et al. 2003).
The diagnostic of Lichen planus is performed by biopsy and/or clinical examination of the mouth or skin lesions (Dorta et al. 2000). The lace-like white streaks that appear bilaterally in the posterior region of the buccal mucosa are virtually pathognomonic. Difficulties in diagnostics may arise if the Lichen planus is superimposed by candidosis lesions, because the organism Candida albicans can change the reticular pattern characteristic of OLP. Thus, the biopsy of the lesions is often necessary to exclude the hypothesis of other erosive or ulcerative diseases such as systemic lupus erythematosus or chronic ulcerative stomatitis.
Histopathological findings are characteristic of Lichen planus, but may not be specific, since other conditions, such as lichenoid reactions to drugs, may also show similar patterns. Varying degrees of parakeratosis and orthokeratosis may be presented on the surface of the epithelium, depending on the characteristics of the injury (erosive or reticular) from which the sample has been removed. The thickness of the spinous layer can also vary. The interpapillary ridges may be absent or hyperplasic, but are classically pointy shaped or “saw-tooth”. The destruction of the basal cell layer of the epithelium (hydropic degeneration) is also evident, accompanied by an intense band-like infiltration, especially T lymphocytes, underlying the epithelium. Degenerate keratinocytes can be seen in the interface area of the epithelium-connective tissue, being called Civatte bodies, colloids or hyaline cytoid (Sugerman et al. 2002). These authors believe that specific and non-specific mechanisms may be involved in the pathogenesis of lichen planus. The former include antigen presentation by keratinocytes of the basal layer and death of antigen-specific cytotoxic T lymphocytes, whereas the latter include mast cell degranulation and activation of matrix metalloproteinases. When these mechanisms are combined, they lead to the accumulation of lymphocytes in the lamina propria underlying the epithelium; disruption of the basement membrane; migration of intraepithelial T lymphocytes; and apoptosis of keratinocytes, which are characteristic of OLP. In addition, the chronic characteristic of the disease can be explained in part by a deficiency in the mechanism of immunosuppression, mediated by transforming beta growth factor.
Regarding the treatment of OLP, 27.6 % of professionals recommend steroids. Some of them reported the use of other therapies for lichen planus reticular with lesions restricted to the oral mucosa, such as cauterization with trichloroacetic acid, rinsing with urea and distilled water, prescription of vitamin A derivatives and tranquilizers (Dorta et al. 2000).
The corticosteroids are the drugs of choice for treatment of lichen planus, because of their ability to modulate the inflammatory and immune response. The topical application and local injection of steroids are being successfully used for controlling the disease. However, the systemic use of corticosteroids is an option for cases presenting severe symptoms. The addition of antifungal drugs improves clinical outcomes. Apparently, this is a consequence of the elimination of secondary growth of Candida albicans in tissues affected by OLP. Antifungal drugs also prevent fungal growth associated to the use of corticosteroids (Dorta et al. 2000).
OLP is a local condition presenting defects of epithelial cells. In these cases, the adherence of Candida to the oral epithelium is the first step in the infection process and enables the yeast to overcome the normal flushing mechanism of body secretions (De Oliveira et al. 2007; Sherman et al. 2002). Candida may act as a secondary pathogen and this super infection can possibly increase the signs and symptoms of OLP, which may be referred as “burning” sensation or discomfort (Kalmar 2007). Dysplastic changes are usually associated to lesions of Candida infection mainly because of the potential endogenous nitrosation of this organism (Sumanth et al. 2003).
Oral Candidosis (OC)
Oral candidosis (OC) is the most frequent oral disease (98 % of cases), being an important nosologic entity of the buccal cavity. It is a fungal infection caused by the pathogenic action of Candida species, most commonly microorganisms C. albicans. These fungi are normally found in the mucous membranes and cause disease only when some growth conditions (predisposing factors) are satisfied. It is estimated that from 30 to 50 % of the population has the microorganism in the mouth and no clinical evidence of infection. The incidence increases with age, reaching close to 60 % of dentate patients over 60 years old (De Oliveira et al. 2007).
According to Stramandinoli (2010), oral candidosis is associated with local and systemic predisposing factors. Local factors comprise poorly sanitized and/or poorly adapted prosthetic restorations or braces; smoking habits; poor oral hygiene and hyposalivation. Systemic predisposing factors include hormonal changes; diseases such as AIDS, diabetes mellitus and other systemic diseases which lead to immunodeficiency; drugs such as corticosteroids, antibiotics and immunosuppressive drugs; and treatments such as head and neck radiotherapy and chemotherapy. Even in the presence of these factors, the invasion of fungus in the superficial layers of the epithelium is mandatory for the disease manifestation.
The candidosis infections are caused by Candida albicans. Other members of the genus Candida, such as C. tropicalis, C. parapsilosis, C. krusei, C. guilliermondii, C. glabrata, C. dubliniensis, can also be found intra-orally, but only occasionally cause disease. The candidosis may present different clinical aspects, complicating the diagnostic. The infection depends on three factors: the immune status of the host, the environment of the oral mucosa and the resistance of C. albicans. The infection can range from mild to fatal when the disease spreads in severely immunocompromised or patients suffering from AIDS (Masaki et al. 2011).
In dental practice, the diagnostic of oral candidosis is usually guided by clinical signs. The definitive identification of the microorganisms is performed by the microbiological diagnosis. For treatment of oral candidosis, various antifungal agents can be used with diverse advantages and disadvantages, as example nystatin, clotrimazole, ketoconazole, fluconazole and itraconazole.
Nystatin, azol-containing therapeutics, e.g. fluconazole, miconazole and ketokonazole, and amphotericin B are the most commonly prescribed drugs for treatment of OC. Alternative substances such as chlorhexidine have both antibacterial and antimycotic effects (Ellepola and Samaranayake 2001b) and colostrum-containing products may have an antimycotic effect as well (Pedersen et al. 2002). C. krusei is natively resistant to azol-containing products and C. glabrata and C. dubliniensis have a weak response to this therapeutics. Thus, selection of resistant Candida species after medical treatment can occur (Isham and Ghannoum 2010). The production of nitrosamine by C. albicans is pointed out as a possible reason for the higher potential for malignization presented by the non-homogeneous leukoplakias in comparison to the homogeneous leukoplakias (Krogh et al. 1987a). In addition to the morbidity, this is the reason for antimycotic treatment in secondary Candida infections in OLP lesions.
Candida is commonly found in oral mucosal lesions and generally causes no problem in healthy people. Overgrowth of Candida, however, can lead to local discomfort, an altered taste sensation, dysphagia from oesophageal overgrowth, resulting in poor nutrition, slow recovery and prolonged hospital stays (Akpan and Morgan 2002).
Association of OLP to OC
Oral candidosis (OC) is subdivided into primary and secondary. Secondary infections are superimposed on other diseases of the oral mucous membranes, such as oral lichen planus (OLP), a chronic inflammatory disease. The histopathological characteristics of OLP include hyperkeratinization (Pindborg et al. 1997), which may contribute to a predisposition for Candida infection. Erythematous or pseudomembranous areas in OLP can be a manifestation of the disease itself or a result of superimposed candidosis. Both cases are associated to morbidity, suffering and pain (Figs. 11.4 and 11.5) (Scully et al. 1998; Williams et al. 2011).
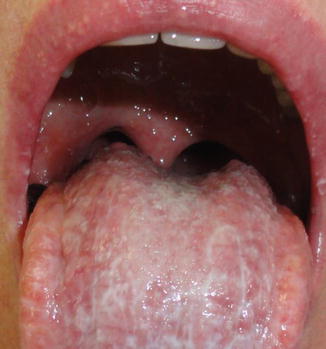
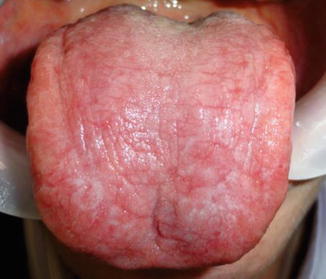

Fig. 11.4
Reticular OLP associated to pseudomembranous candidosis in the tongue

Fig. 11.5
Case of Fig. 11.4 after treatment with nystatin (100,000 IU) three mouthwashes per day during 2 weeks. Note a significant clinical improvement
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


