Note: For further discussion please see Volume I, Chapter 9 : Chronic Maxillomandibular and Head and Neck Pain by John M. Gregg.
According to the National Health Interview Survey (NHIS) conducted by the Centers for Disease Control and Prevention (CDC) in 2005, approximately 7% of Americans suffer from facial pain each year. The term facial pain may be used to describe any unpleasant, annoying, or descriptive sensation in the facial region, including those emanating from the oral cavity, dentition, maxilla, mandible, sinus, orbital, cranial, and nasal regions of the head and neck. While it is clear that facial pain is mediated primarily by nerve fibers of trigeminal nerve origin, other sensory nerves and nerve fibers may also play a role in facial pain transmission. In order to understand facial pain, one must first understand the neurophysiology of pain: from the release of pain-producing neurotransmitters and the interaction with receptors on peripheral nerve fibers innervating head and neck structures to the complex cortical reorganization and processing that occurs in chronic pain. This chapter will highlight the key neurophysiologic mechanisms that underlie both acute and chronic facial pain entities.
Chronic facial pain is one of the most challenging entities for a clinician to diagnose and treat. It is usually of greater than 6 months’ duration, unrelenting, and recalcitrant to conventional modes of pharmacotherapy and/or surgical intervention. The chronic pain patient who relies on chronic opioid therapy poses a particular challenge to the oral and maxillofacial surgeon from an intraoperative and postoperative pain management standpoint. This chapter will discuss differential diagnoses of chronic facial pain, treatment recommendations, and barriers that currently exist for the management of the chronic facial pain patient. Identifying the barriers and eliminating them through discussions with the patient and health care providers will allow for effective pain management and abolition of biases that currently affect many chronic pain sufferers.
Facial pain, like other types of pain, can be complex and multidimensional and therefore clinical evaluation becomes an important aspect in the diagnosis of the facial pain patient. Many clinical specialties play a role in the diagnosis and management of facial pain. These include anesthesiologists, dentists, endodontists, oral and maxillofacial surgeons, facial pain experts, otolaryngologists, neurologists, neurosurgeons, physical therapists, psychiatrists, psychologists, and chemical dependency physicians. In complex cases of facial pain, a team approach is usually taken, resulting in improved therapeutic outcomes. With an understanding of the complex neurophysiology that exists in the facial pain patient, an idea of how to delineate the correct facial pain diagnosis, and an understanding of the alternative treatment modalities that currently exist, the clinician will be enabled to manage one of the most complex and challenging pathologic entities—a patient with chronic facial pain.
NEUROPHYSIOLOGY OF PAIN
TRIGEMINAL ANATOMY
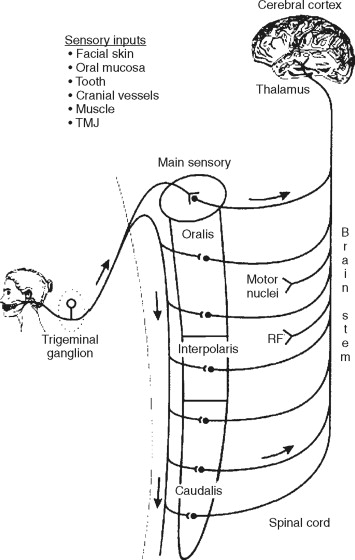
A thorough understanding of neuroanatomy and neurophysiology is imperative to fully appreciate the complex nature of facial pain. While it is clear that other sensory fibers are important in the transmission of painful information from the head and neck (e.g., cervical spinal nerves, sympathetic fibers), the majority of pain in the head and neck is transmitted via branches of the fifth cranial nerve—the trigeminal nerve. The trigeminal nerve is considered the largest of the cranial nerves. The primary sensory neurons (also known as first-order neurons) of the trigeminal nerve are located within the largest ganglia in the human body—the trigeminal ganglia. The trigeminal ganglia are housed within the petrous portion of the temporal bone in the middle cranial fossa in close proximity to the internal carotid artery and cavernous sinus. It is considered a bipolar neuron in that its primary afferent (peripheral nerve) fibers terminate within the head and neck while its central fibers terminate within the medullary dorsal horn and cervical spinal cord. Its extensive distribution highlights the amount of sensory input the head and neck receives.
The trigeminal sensory fibers synapse with second-order neurons located within the spinal trigeminal nuclear complex. The trigeminal brainstem nuclear complex is subdivided into the (1) chief main sensory nucleus, (2) spinal trigeminal nucleus oralis, (3) spinal trigeminal nucleus interpolaris, and (4) spinal trigeminal nucleus caudalis. The spinal trigeminal nucleus caudalis has been implicated as the main nucleus that receives painful or nociceptive input from peripheral targets. There is a significant convergence of non–trigeminal nerve fibers arising from the cervical spine and vagus and glossopharyngeal nerve terminals onto trigeminal nuclei. The large convergence of cranial and spinal nerves explains the presence of referred pain to non–head and neck structures in the facial pain patient.
Neurons within the spinal trigeminal sensory nuclear complex project to higher brain centers such as the thalamus and reticular formation . The pathways are known as the trigeminothalamic and trigeminoreticular tracts, respectively, and they project to higher order neurons located within the somatosensory cortex and anterior cingulated cortex. The processing of both discriminative (quality, severity, and location) and affective (emotional) components of pain occurs at these cortical levels ( Figure 52-1 ).
PERIPHERAL MECHANISMS OF PAIN
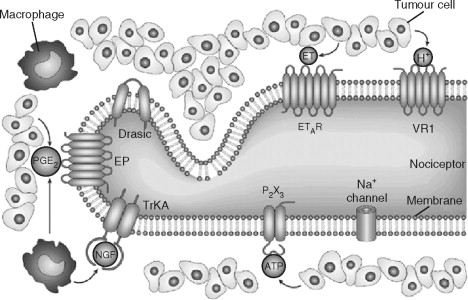
Considerable advances have been made in the past 2 decades in identifying cellular and molecular mechanisms involved in facial pain transmission. Injury to peripheral tissues certainly occurs during oral and maxillofacial surgical procedures. Pain generally occurs during tissue damage and is a result of release of neurotransmitters, cytokines, and factors from damaged cells, adjacent blood vessels, and nerve terminals. Prostaglandins, substance P, calcitonin gene–related peptide (CGRP), bradykinins, histamine, interleukin-1 and interleukin-6, and serotonin are examples of proinflammatory mediators that are involved in the generation of the inflammatory state that accompanies tissue destruction.
Pain transduction occurs when these proinflammatory mediators interact with channels and receptors expressed on the peripheral nerve membrane. These are specialized structures that detect a diversity of chemical and thermal stimuli ( Figure 52-2 ). During minor tissue damage, proinflammatory mediators are released and bind to their respective receptor or channel, and pain transduction occurs and resolves over time. Peripheral sensitization occurs when the peripheral nerve becomes sensitized because of constant stimulation and activation. This may be in the form of decreased excitation thresholds, up-regulation of receptors and/or channels in both nerve terminals and sensory neurons, or recruitment of previously silent pain receptors or nociceptors.
NEUROPHYSIOLOGY OF PERIPHERAL NERVE FIBERS
Peripheral nerve fibers, also known as primary afferent nerve fibers, are generally classified according to degree of myelination and conduction velocities. The A beta (Aβ) fibers are myelinated and have fast conduction velocities (35-120 m/sec). These fibers transmit mechanosensory information and have specialized terminals called Meissner’s, pacinian, Merkel’s, and Ruffini’s corpuscles located within skin. Although this fiber type does not transmit painful information, current studies have now demonstrated their role in chronic pain states. Nerve fibers that transmit pain and thermal information are known as A delta and C fibers and are lightly myelinated and unmyelinated, respectively. The A delta (Aδ) fibers have relatively fast conduction velocities (5-30 m/sec) when compared to C fibers (0.5-2 m/sec). Both Aδ and C fibers terminate in skin, bone, muscle, and joints as free nerve endings.
Peripheral nerve fibers that innervate the head and neck arise from the ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions of the trigeminal nerve (cranial nerve V [CN V]) as well as cutaneous inputs from cervical spinal nerves as far caudal as spinal C7. The extensive rostrocaudal input of painful information from the head and neck as well as significant convergence of input from other cranial and spinal nerves (e.g., CN X, CN XII, cervical spinal nerves) may in part explain the referral patterns in the chronic pain patient.
CENTRAL MECHANISMS OF PAIN
Centrally mediated pain is defined as pain initiated or caused by a primary lesion or dysfunction in the central nervous system. While it is rare in practice to see patients with primary CNS lesions, it is not uncommon for chronic facial pain patients to be first evaluated by an oral and maxillofacial surgeon. The primary mechanism for chronic pain lies in neuroplastic changes that occur within the central nervous system in response to chronic painful stimuli. Much of the research in central pain mechanisms is related to other chronic pain conditions such as traumatic neuropathy and diabetic neuropathy in both animal and human models. Certainly animal models of chronic facial pain exist that have increased our understanding of the pathophysiology of centrallymediated chronic facial pain.
CENTRAL SENSITIZATION
Central sensitization refers to the heightened reactivity of central nervous system neurons in the face of sustained peripheral neural input. While this may occur within the thalamus and cortex, research has been primarily focused on the dorsal horn of the spinal cord. Electrophysiologic and anatomic studies have demonstrated a change in the activity and responsiveness of dorsal horn neurons in response to persistent painful stimulation.
Persistent stimulation of unmyelinated C fibers results in the increased activity and responsiveness of spinal neurons that receive input from the stimulated unmyelinated C fiber. This increased responsiveness is of short (10 sec) duration and is known as wind up . Sensitization can also occur when persistent stimulation results in phenotypic changes in neurons that do not receive persistent painful stimulation, but are adjacent to neurons that do receive painful input. Typically these neurons receive input from Aβ fibers that normally do not transmit painful stimuli. However, once sensitized, these neurons are capable of transmitting both nonpainful and painful information. Central sensitization is mediated in part by glutamate, substance P, prostaglandins, and growth factors. These receptors/ion channels are known as N- methyl- d -aspartate (NMDA), neurokinin-1 (NK-1), EP, and tyrosine kinase B (trkB), respectively. Up-regulation of transient receptor potential (TRPV1) and sodium channels has also been reported in central sensitization. Pain evoked from a stimulus that normally does not evoke pain (allodynia) may result from central sensitization.
DESCENDING INHIBITORY CONTROL
A protective mechanism exists in which adrenergic and serotoninergic fibers arising from the hypothalamus and periaqueductal gray matter descend and terminate in the medulla and superficial dorsal horn. This descending pathway controls or dampens painful sensory input from primary afferent fibers arising from the periphery. Chemical denervation of the inhibitory pathway results in an increased response to a stimulus that is normally painful (hyperalgesia). Inhibitory receptors have also been shown to be reduced in animal models of chronic pain. Thus disruption of inhibitory pathways that are normally tonically active may also result in chronic pain.
The constant barrage of neurochemicals that are implicated in pain transmission results in changes in the activity and phenotype of nerve fibers and neurons within the peripheral and central nervous system. These changes result in a chronic and debilitating condition that is recalcitrant to many conventional modes of acute pain therapy. The chronic pain patient has a “pain portrait” that is distinct from the acute pain patient and its management reflects this difference. However, not all chronic facial pain entities are alike. Diagnosis of the facial pain patient requires meticulous investigation and educated elimination of other pain entities.
CENTRAL MECHANISMS OF PAIN
Centrally mediated pain is defined as pain initiated or caused by a primary lesion or dysfunction in the central nervous system. While it is rare in practice to see patients with primary CNS lesions, it is not uncommon for chronic facial pain patients to be first evaluated by an oral and maxillofacial surgeon. The primary mechanism for chronic pain lies in neuroplastic changes that occur within the central nervous system in response to chronic painful stimuli. Much of the research in central pain mechanisms is related to other chronic pain conditions such as traumatic neuropathy and diabetic neuropathy in both animal and human models. Certainly animal models of chronic facial pain exist that have increased our understanding of the pathophysiology of centrallymediated chronic facial pain.
CENTRAL SENSITIZATION
Central sensitization refers to the heightened reactivity of central nervous system neurons in the face of sustained peripheral neural input. While this may occur within the thalamus and cortex, research has been primarily focused on the dorsal horn of the spinal cord. Electrophysiologic and anatomic studies have demonstrated a change in the activity and responsiveness of dorsal horn neurons in response to persistent painful stimulation.
Persistent stimulation of unmyelinated C fibers results in the increased activity and responsiveness of spinal neurons that receive input from the stimulated unmyelinated C fiber. This increased responsiveness is of short (10 sec) duration and is known as wind up . Sensitization can also occur when persistent stimulation results in phenotypic changes in neurons that do not receive persistent painful stimulation, but are adjacent to neurons that do receive painful input. Typically these neurons receive input from Aβ fibers that normally do not transmit painful stimuli. However, once sensitized, these neurons are capable of transmitting both nonpainful and painful information. Central sensitization is mediated in part by glutamate, substance P, prostaglandins, and growth factors. These receptors/ion channels are known as N- methyl- d -aspartate (NMDA), neurokinin-1 (NK-1), EP, and tyrosine kinase B (trkB), respectively. Up-regulation of transient receptor potential (TRPV1) and sodium channels has also been reported in central sensitization. Pain evoked from a stimulus that normally does not evoke pain (allodynia) may result from central sensitization.
DESCENDING INHIBITORY CONTROL
A protective mechanism exists in which adrenergic and serotoninergic fibers arising from the hypothalamus and periaqueductal gray matter descend and terminate in the medulla and superficial dorsal horn. This descending pathway controls or dampens painful sensory input from primary afferent fibers arising from the periphery. Chemical denervation of the inhibitory pathway results in an increased response to a stimulus that is normally painful (hyperalgesia). Inhibitory receptors have also been shown to be reduced in animal models of chronic pain. Thus disruption of inhibitory pathways that are normally tonically active may also result in chronic pain.
The constant barrage of neurochemicals that are implicated in pain transmission results in changes in the activity and phenotype of nerve fibers and neurons within the peripheral and central nervous system. These changes result in a chronic and debilitating condition that is recalcitrant to many conventional modes of acute pain therapy. The chronic pain patient has a “pain portrait” that is distinct from the acute pain patient and its management reflects this difference. However, not all chronic facial pain entities are alike. Diagnosis of the facial pain patient requires meticulous investigation and educated elimination of other pain entities.
EVALUATION OF THE FACIAL PAIN PATIENT
The chronic facial pain patient poses one of the most challenging cases to the oral and maxillofacial surgeon. One major reason is because pain is primarily subjective in nature. It is difficult to measure pain objectively as it cannot be seen nor is it palpable on clinical exam. The clinician is required to gather clinical and historical information, formulate a list of differential diagnoses, and systematically eliminate least likely entities until a final diagnosis is achieved. To formulate a list of differential diagnoses assumes that the most common and simplest forms of pain have been eliminated. A thorough assessment of the patient’s history of present illness can be obtained by asking pointed questions that may identify the etiology or etiologies of the patient’s facial pain. Questions regarding incidence, character, and duration as well as modalities to treat pain may also assist in the diagnosis of facial pain. The key to successful diagnosis of the chronic facial pain patient lies in identifying and answering key questions to develop a list of differential diagnoses.
CHIEF COMPLAINT
The patient’s chief complaint should always be documented in his/her own words with the use of parentheses. The severity of the patient’s pain at time of examination should also be documented. The most practical scale used is the numeric pain rating scale (NPRS) where the patient verbalizes a number corresponding to the extent of his/her pain: 0 is no pain and 10 is the worst pain imaginable.
HISTORY OF PRESENT ILLNESS
Pain onset and duration are important to determine whether the onset was acute in nature or developed gradually over time. This is a particularly challenging task with most facial pain patients. In general, there is an association made with an event, procedure, or circumstance that may not have a role in the generation of the symptoms. If the facial pain is acute, one must further question the events surrounding its onset. A history of previous surgical and non-surgical therapies to treat facial pain must also be elucidated to establish a diagnosis, assess prognosis, and define a surgical plan if indicated.
The quality, character, and radiation of pain should be assessed in the facial pain patient. Diffuse or localized pain will identify whether the pain is isolated to a single anatomic entity or whether multiple structures are involved. Descriptions of pain character such as “dull and throbbing” or “sharp and stabbing” will help to identify what dominant peripheral nerve fiber type is involved, which will assist in determining type of facial pain entity. Facial pain may radiate or be referred to adjacent muscular structures and the forehead, suggesting generalized myofacial pain disorder.
The patient should be asked about analgesics used in attempts to alleviate facial pain and whether these analgesics are efficacious. The chronic use of narcotics, spasmolytics, and antidepressants to manage facial pain is typically seen in patients with a pre-existing diagnosis of chronic facial pain. Questions should be directed towards identifying therapies or medications that relieve pain. For example, if nonsteroidal anti-inflammatory drugs and heat packs relieve pain, the patient likely suffers from mild inflammation of the muscle and/or joint. Habits should also be assessed to determine if the etiology of facial pain arises from parafunctional habits such as repetitive jaw motion or extreme jaw forces from gum chewing or clenching, respectively.
PAST MEDICAL HISTORY RELATED TO THE FACIAL PAIN PATIENT
The review of a patient’s medical history is always important; however, it is clear that certain systemic conditions are commonly observed in the chronic facial pain patient. It is important to determine whether anxiety, depression, and/or other psychogenic disorders are present and appropriately managed. Clearly, facial pain patients with concomitant depression derive benefit from antidepressant medication. Fibromyalgia is another chronic pain entity that affects 4% of Americans. It is generally associated with diffuse generalized pain and fatigue and occurs in approximately 15% to 20% of facial pain patients. Other systemic disorders that are commonly observed in the chronic facial pain patient include autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, diabetes and other conditions resulting from chronic insulin deficiency, and also neurologic disorders such as multiple sclerosis. The concomitant co-morbidities of systemic disease and chronic facial pain add to the complexity of the diagnosis and management of this challenging condition.
CLINICAL EXAMINATION
A thorough dental examination must be performed to rule out odontogenic sources of pain and avoid unnecessary dental treatments ( Figure 52-3 ). In most clinical practice, the referring general dentist or clinician will have diagnosed the most common entities that cause pain: dental caries and infection. Facial pain may be referred from teeth and/or supportive structures and therefore pathology associated with such structures must be ruled out.
The most common and frequently seen pathology that results in facial include the following:
- •
Tooth fractures
- •
Caries
- •
Temporomandibular disorders (TMDs)
- •
Salivary gland disease
- •
Ulcers
- •
Neoplasms
- •
Infection
While the clinical examination and radiologic findings associated with these pathologies are beyond the scope of this chapter, it is important to exclude these entities to obtain the diagnosis of chronic facial pain. In addition, patients with no observable etiology of facial pain cannot be dismissed as hypochondriacs. Some insidious neoplasms, both benign and malignant, develop slowly. Pain may be the first sign that a neoplasm is present before progressing to a size that is detectable by either clinical evaluation or imaging.
The clinical examination of the chronic facial pain patient is very challenging as there is no visible entity that can easily be seen or felt. Instead, the clinical examination should focus on the following aspects to derive a list of differential diagnoses:
- •
Location
- •
Identify etiology of pain by location (sensory nerve innervation).
- •
Does the pain arise from dental, gingival, or dentoalveolar structures?
- •
Does the pain arise from stimulation of a specific sensory nerve distribution?
- •
Is the pain limited to a specific sensory distribution?
- •
Does the pain radiate to other structures within or beyond the head and neck?
- •
Do the involved structures demonstrate abnormalities in color, temperature, turgor, or sensation?
- •
- •
Duration
- •
Identify whether the pain is intermittent or constant.
- •
If spontaneous, identify the type of stimuli that causes the pain.
- •
Is the pain sharp and stabbing when stimulated?
- •
Is the pain reproducible and consistent?
- •
Does the pain spontaneously resolve?
- •
- •
Character
- •
Identify the general pain sensation.
- •
Is the pain sharp, dull, or lancinating?
- •
Is the pain a burning sensation?
- •
Are there any sensations other than pain that coexist (for example, tingling, numbness, formication)?
- •
- •
Presence of sensory or motor deficits
- •
Quantitative clinical neurosensory testing must be performed for all patients with documented sensory deficits.
- •
Level A testing assesses spatiotemporal sensory discrimination using two methods: brush stroke directional testing and two-point discrimination. Both methods assess the discriminatory competency of myelinated Aβ fibers.
- •
Level B testing assesses static sensory discrimination using Semmes-Weinstein nylon monofilaments of graded thicknesses. Electronic versions are available.
- •
Level C testing assesses sharp-dull discrimination and pain thresholds. Specialized equipment such as calibrated thermodes or pain algometers are available although a sharp instrument (e.g., needle) may be used. This level of testing assesses the competency of unmyelinated C fibers and lightly myelinated Aδ fibers.
- •
PAIN DESCRIPTORS
A sensory map should be developed based on the clinical neurosensory examination. In unilateral cases, both sides of the head and neck should be evaluated; the contralateral side may serve as an internal control. Based on the clinical findings and patient interview, the facial pain entity may now be described using conventional pain terminology ( Table 52-1 ). Following the patient interview and clinical examination, the clinician is now able to describe the facial pain entity. Utilization of appropriate terminology will improve communication between health care professionals, assist in formulating a list of differential diagnoses, and assess the progress of both the pain condition and the treatment rendered.
| Type of Pain | Definition |
|---|---|
| Allodynia | Pain evoked with stimulus that normally does not provoke pain |
| Analgesia | Absence of pain in response to stimulation that would normally be painful |
| Arthralgia | Pain within a joint |
| Causalgia | A syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion, often combined with vasomotor and sudomotor dysfunction and later trophic changes |
| Central pain | Pain initiated or caused by a primary lesion or dysfunction in the central nervous system |
| Dysesthesia | An unpleasant abnormal sensation, whether spontaneous or evoked |
| Hyperesthesia | Increased sensitivity to stimulation |
| Hypoesthesia | Diminished sensitivity to stimulation |
| Hyperalgesia | An increased response to a stimulus that is normally painful |
| Hypoalgesia | Diminished sensitivity to noxious stimulation |
| Hyperpathia | A painful syndrome, characterized by increased reaction to a stimulus, heightened with repetition; hyperpathia may occur with hyperesthesia, hyperalgesia, or dysesthesia |
| Neuralgia | Pain in distribution of nerve or nerves |
| Neuropathic pain | Pain initiated or caused by a primary lesion or dysfunction in the nervous system |
| Neuropathy | A disturbance of function or pathologic change in a nerve: in one nerve, mononeuropathy; in several nerves, mononeuropathy multiplex; if diffuse and bilateral, polyneuropathy |
| Paresthesia | An abnormal sensation, whether spontaneous or evoked |
IMAGING MODALITIES
Appropriate imaging studies may be necessary to allow the clinician to derive a list of differential diagnoses and exclude certain conditions. Depending on the source, character, and complexity of pain, multiple imaging modalities may be considered. While it is appropriate to consider imaging studies, chronic facial pain is difficult to diagnose with imaging studies as there is a lack of definitive abnormalities that are characteristic or pathognomonic for facial pain.
- •
Orthopantomogram: The orthopantomogram is the most common screening film used in the oral and maxillofacial surgery practice. It is both economical and practical in its use and provides minimum radiation exposure to the patient. Additionally, patients often have panoramic radiographs that predate the consultation visit or date of onset of pain that can be used as a reference or baseline image. While panoramic imaging may not establish the clinical diagnosis, it has its utility as a screening tool before requesting more advanced imaging.
- •
Computed tomographic (CT) imaging: Clinical examination will dictate whether advanced imaging is warranted. Although CT imaging is not a standard modality for chronic pain, newer techniques that use CT technology are being used for facial pain patients. The role of single photon emission computed tomography with technetium 99m methylene diphosphonate (SPECT with 99mTc-MDP) and computed tomography (CT) to diagnose facial pain has recently been reported. This technology uses radiolabeled technetium to identify areas of increased bone metabolism and uses diphosphonates, which display strong affinity to hydroxylapatite crystals within bone. Using SPECT technology, data are acquired in all three planes (axial, coronal, and sagittal planes) to allow for three-dimensional mapping. When used in conjunction with conventional CT data, functional anatomic mapping is obtained, which allows for three-dimensional rendering. Advantages include (1) low radiation requirements (0.00675 Gy per 70-kg adult) and (2) high sensitivity. Disadvantages include (1) need for specialized equipment and staff, (2) cost, and (3) poor specificity. SPECT in conjunction with CT is currently being used in oncology as a surveillance tool.
- •
Functional magnetic resonance imaging (fMRI): Functional magnetic resonance imaging is an indirect method used to assess neural activity within the central nervous system. It utilizes the BOLD effect, or changes in blood oxygenation, by quantifying the relative concentrations of oxyhemoglobin and deoxyhemoglobin. An increase in blood flow and, hence, neural activity results in a decrease in deoxyhemoglobin level. These changes are then mapped onto previously obtained anatomic maps of the patient’s brain. Functional MRI has been used in studies of headache, acute dental pain, trigeminal neuropathy, and atypical facial pain to examine areas within the cortex that are involved in pain processing. Both discriminative and affective components of pain have been mapped to the somatosensory cortex and anterior cingulate cortex, respectively, based on fMRI studies. While its utility in examining treatment success has been reported, its diagnostic capabilities remain limited because of a relative paucity of neuroimaging studies on trigeminal pain pathways.
DIFFERENTIAL DIAGNOSIS
After establishing the character, duration, quantity, and quality of the facial pain entity, the clinician must eliminate more common facial pain causation or etiology. Then, augmenting information gleaned from the patient interview and exam with appropriate imaging modalities, the clinician is now able to derive a list of differential diagnoses of chronic facial pain entities. While the differential diagnoses are only a partial listing of all chronic facial pain states, these are the most commonly encountered and the most challenging to diagnose.
COMPLEX REGIONAL PAIN SYNDROME (CRPS)
The diagnosis of CRPS is based almost exclusively on clinical examination. Two types of CRPS exist: type I or reflex sympathetic dystrophy (RSD) and type II or causalgia. Based on the 2005 consensus meeting of the International Association for the Study of Pain (IASP), the diagnosis of CRPS can be made if the following criteria are fulfilled:
- •
The presence of a noxious initiating event without nerve damage (type I: reflex sympathetic dystrophy) or with nerve damage (type II: causalgia)
- •
Spontaneous pain or hyperalgesia that is disproportionate to the inciting event and that extends beyond a single nerve territory
- •
Autonomic dysfunction: edema, cutaneous blood flow changes (increased or decreased), motor changes, and/or trophic changes
- •
All other diagnoses are excluded.
- •
Type I (reflex sympathetic dystrophy): Nearly 75% of patients with CRPS type I or RSD will demonstrate pain that is described as aching, burning, or pricking in nature. Sometimes the pain can be “shooting” in character. All will describe some type of hyperalgesia to innocuous mechanical stimuli that may extend beyond the site of injury. This finding highlights the central sensitization that occurs in RSD. Muscle weakness or tremors may be observed in as high as 75% of RSD patients. Autonomic disturbances, such as edema and thermal cutaneous dysregulation, and trophic changes, such as disruptions in hair growth and skin atrophy, are seen in more severe cases of RSD.
- •
Type II (causalgia): CRPS II or causalgia is distinguished from RSD if nerve damage is present. Similar to other neuropathic pain conditions, pain resulting from causalgia is described as electrical or shooting pain. Some patients may also exhibit areas of simultaneous hypoesthesia and extreme allodynia. Autonomic and motor disturbances similar to those seen in RSD are present.
Management of CRPS is limited to pharmacotherapy associated with the management of neuropathic pain conditions. These include tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), anticonvulsants, gabapentin, steroids, and opioids. Sympathetic nerve blocks and topical local anesthetics have also been reported with mixed success.
NEURALGIA-INDUCING CAVITATIONAL OSTEONECROSIS (NICO)
Since first described in 1979, the concept of NICO has been questioned by some clinicians. NICO has been defined as a cavitation within maxillary and/or mandibular alveolar bone in an area of previous trauma (e.g., dental extraction or endodontic therapy). The pathophysiology of NICO has been suggested to arise in defects in genes that are responsible for coagulation. These coagulopathic states may result in marrow ischemia similar to that seen in avascular necrosis of the femur. Marrow ischemia results in nonhealing extraction sites that cavitate and, in some cases, form colonies of bacteria, resulting in osteomyelitis. Patients with NICO complain of continuous or paroxysmal pain that is sharp, stabbing, burning, and lancinating in character. The location of pain is primarily at the site of initial injury and may spread to a larger area, often crossing midline structures.
Treatment of NICO lesions has been primarily exploration and debridement of cavitary lesions and long-term management with either gauze-impregnated or injectable antibiotics for up to 9 weeks’ duration. Approximately 66% of patients report decreases in pain after surgery; however, recurrence in pain symptoms is often seen. Management of NICO with warfarin and stanozolol has been reported; however, pain relief and side effect profiles limit their utility.
ATYPICAL FACIAL PAIN
Atypical facial pain is a diagnostic category that includes both atypical odontalgia and stomatodynia. It is primarily a diagnosis of exclusion that is based primarily on history and clinical examination. Like many chronic facial pain entities, atypical facial pain cannot be diagnosed based on laboratory and/or radiologic findings as these are typically normal.
- •
Atypical odontalgia: Atypical odontalgia (AO) is one of the most challenging conditions an oral and maxillofacial surgeon will face. To fulfill the diagnostic criteria for atypical odontalgia, the following conditions must be met:
- •
Pain is located in the mouth or face.
- •
Pain is described as constant, deep, and dull ache.
- •
- •
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


