Fig. 9.1
Schematic diagram of thermal CVD (a), plasma enhanced CVD (PECVD) (b) and laser CVD (LCVD) (c)
PECVD (Fig. 9.1a) uses plasma as an auxiliary energy source. An electromagnetic field with radio frequency (RF: 13.5 MHz) or micro-wave (2.45 GHz) can be applied to a deposition zone to form the plasma. The gas can be discharged and dissociated to activate ions, radicals and electrons. These activated species are significantly reactive, even at low temperatures, forming non-equilibrium or quasi-equilibrium films [10]. The authors first utilized PECVD for preparing bio-ceramic coatings as shown later.
Lasers can be an auxiliary energy source of light and heat in CVD, and thus LCVD (Fig. 9.1c) can be categorized into two types: photolytic LCVD and pyrolytic LCVD [6]. Since a source gas may absorb a specific laser wavelength, photolytic LCVD can prepare films without substrate heating. The laser passes through a gas phase, directly decomposing source gases. Photolytic LCVD using a high energy laser, typically an ultra-violet or Excimer laser, has the advantage of low temperature deposition without thermal degradation of the substrate. However, photolytic LCVD cannot create a wide-area coating at a high deposition rate. In pyrolytic LCVD, infra-red lasers, such as CO2 and Nd:YAG lasers, are generally used. Pyrolytic LCVD heats locally at a small area of the substrate by focusing the laser beam; thus, source gases can easily access the heated area. The deposition rate of pyrolytic LCVD can be significantly high, reaching several 100 m/h [6]. However, the deposition area is usually less than several mm2. Therefore, pyrolytic LCVD are generally understood to not make large-area coatings on substrates with complicated shape. The authors first developed LCVD to prepare oxide and non-oxide films at high deposition speeds (more than several 100 μm/h) on wide-area substrates (around several cm2) by using a high power laser (several 100 W of CO2, Nd:YAG and diode lasers), as shown later [11, 12].
9.3 CVD of Ca–P–O Films and Their Bio-Characteristics
Figure 9.2 depicts the phase diagram of a Ca–P–O system [13], which contains various bio-ceramic materials. α- and β-Ca3P2O8 (TCP: tricalcium phosphate) have been widely studied as bio-resorbable materials. Figure 9.3 depicts the crystal structures of α- and β-TCP. The structure of α-TCP (Fig. 9.3a) is classified as a glaserite-type structure, where Ca ions exhibit coordination numbers ranging from five to nine and share edges with a PO4 group [14]. Ca and phosphate ions are packed in columns along the c-axis in two ways; one contains only cations and the other contains both cations and anions. While the α-TCP is thermo-dynamically stable at 1,393–1,743 K and metastable at room temperature, β-TCP is stable below 1,393 K. The structure of β-TCP (Fig. 9.3b) is related to that of Ba3(VO4)2, although β-TCP has lower symmetry due to site vacancies and distortions [15]. Ca ions coordinated to six, seven, eight and nine oxygen ions share edges with PO4 tetrahedra. The major difference in crystal structure between α- and β-TCP is that β-TCP has no cation–cation columns. Additionally, compared to that of β-TCP, α-TCP has a higher internal energy because of its higher volume per formula unit, which is consistent with α-TCP being the high-temperature phase [14].
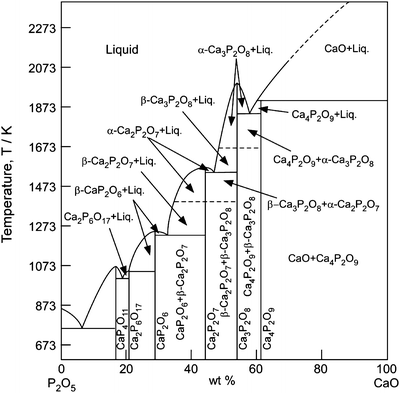
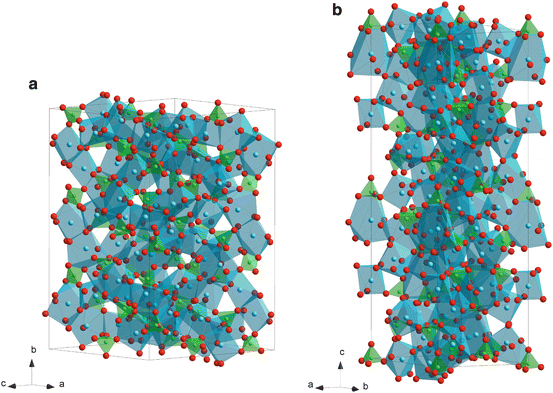

Fig. 9.2
A phase diagram of Ca–P–O system

Fig. 9.3
Crystal structures of α- (a) and β-tricalcium phosphate (TCP) (b)
α- and β-Ca2P2O7 (CPP: calcium pyrophosphate) have been scarcely studied. The crystal structures of α- and β-CPP are illustrated in Fig. 9.4a, b, respectively. Structures of both α- and β-CPP contain pyro-groups, P2O7, which consist of two corner-shared PO4 tetrahedra with P–O–P angles of 130° for the α-phase and angles of 131° and 135° for the β-phase [16, 17]. In α-CPP, Ca ions coordinates with eight oxygen atoms and the chains of edge-shared Ca polyhedra form sheets parallel and perpendicular to the ac plane. The coordination numbers of Ca in β-CPP are seven, eight and nine; each pyrophosphate group is linked by commonly-shared Ca atoms, forming infinite pyrophosphate-Ca chelate-like chains [17]. Allen et al. prepared an α-CPP film by thermal CVD using Ca(dpm)2 (dpm: dipivaloylmethanate) and P2O5 at a total pressure (P tot) of 1 kPa and a deposition temperature of 1,123 K. The β-CPP film was heat-treated at 1,623 K and transformed to a β-TCP film [18].
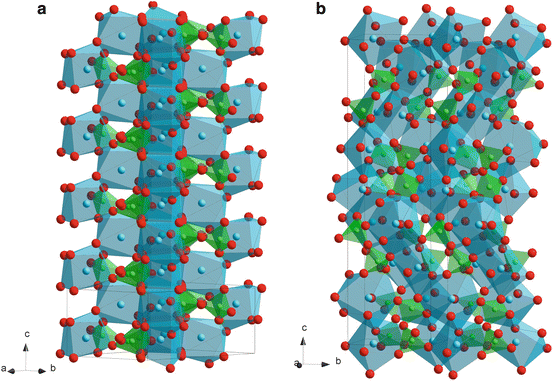

Fig. 9.4
Crystal structures of α- (a) and β-calcium pyrophosphate (CPP) (b)
Ca4P2O9 (TTCP: tetracalcium phosphate) would be more bio-resorbable than TCP because of its greater P2O5 content. TTCP is also written as Ca4(PO4)2O (tetracalcium diphosphate monooxide) since its structure contains Ca ions coordinated with seven and eight oxygen atoms that share PO4 edges, and oxygen ions strongly coordinated to four Ca ions, forming tetrahedra of OCa4, as oxide ions (Fig. 9.5a) [19]. The Ca and P atoms lie near four sheets that contain two cation–anion (Ca–PO4) columns and one cation–cation (Ca-Ca) column perpendicular to the b-axis. Because of these so-called ‘apatitic layers’, TTCP has a close structural relationship with calcium hydroxyapatite (HAp: Ca10(PO4)6(OH)2) and its dehydration product, calcium oxyapatite (OAp: Ca10(PO4)6O) [19–22].
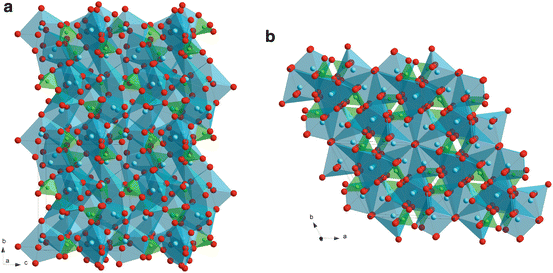

Fig. 9.5
Crystal structure of tetracalcium phosphate (TTCP) (a) calcium hydroxyapatite (HAp) (b)
Figure 9.5b depicts a crystal structure of HAp, which is illustrated by tetrahedra of PO4 and polyhedra of Ca ions coordinated with seven and nine oxygen atoms. HAp and OAp have ‘apatite layers’, composed of two Ca-PO4 columns and one Ca-Ca column parallel to the ac plane, and hydroxyl ions in HAp and oxygen ions in OAp are located at the center of Ca hexagons parallel to the ab plane. HAp or OAp have been frequently studied because HAp is bio-active and similar to human bones. Although many techniques including solid-state sintering, sol–gel and magnetron sputtering [23] have been employed to prepare OAp or HAp, Darr et al. prepared fluorine-containing carbonated hydroxyapatite by thermal CVD using Ca(tmhd)2 (where tmhd=2,2,6,6,-tetramethylheptane-3,5-dione) and P2O5 [24]. The crystal structure of the film was not investigated, but the Ca/P content of the film was about 1.3 which is different from that of HAp (Ca/P = 1.7). The bio-compatibility of this film was not reported. Since OH is easily evaporated in a vacuum, preparing OH-containing HAp by dry processes (vacuum processes), such as CVD and sputtering, is difficult. OAp film prepared by magnetron sputtering did not contain OH [25
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


