(1)
Department of Endodontics, New York University College of Dentistry, New York, NY, USA
Abstract
The most common cause of pulp/periapical pathosis is caries. In this chapter host–microbial interaction and the pulp’s dynamic response to the inflammatory process is described. The process occurs within a unique low-compliance environment due to the hard unyielding walls of dentin surrounding the pulp. The result of this anatomy is an inability of the pulp to swell as it becomes progressively more inflamed. Understanding canal anatomy is an essential part of recognizing endodontic symptoms and providing emergency pain relief and subsequent therapy.
Recognizing factors that predispose a patient to pain is an important part of implementing pain-preventive strategies. While iatrogenic errors (inaccurate measurement control, over-instrumentation) often play a role in postoperative pain, there are other, less obvious, factors including genetics, gender, and anxiety that may predispose a patient to pain. The significance of these factors in relation to patient’s pain is described. Strategies to reduce patient’s anxiety are also discussed.
Postoperative pain may occur following instrumentation or obturation. Pain-preventive, evidence-based strategies, including occlusal reduction, are described.
5.1 Causes of Endodontic Pain
Prior to discussing pain-preventive strategies, it is essential to understand basic pulp biology and the pulp’s response to caries and other irritants.
Pulpal disease and concomitant pain is caused by caries, trauma, or as a result of restorative procedures. Although there are differences in the disease processes, ultimately microbiological factors are of critical importance.
Although caries is not the only cause of pulpal disease, understanding the principles of the pulp’s dynamic response to caries is an important step in developing a pain-preventive strategy. Onset of pain subsequent to caries varies depending on a number of factors including presence of prior restorations, individual pain thresholds and host inflammatory and immune resistance. The response to advancing caries is so varied that even a carious exposure may occur without pain and is classified as asymptomatic irreversible pulpitis.
5.2 Caries
Even at early stages of the carious process, pulpal inflammation is seen in the pulp. Dentin permeability permits ingress of bacterial toxins into the pulp long before there is actual exposure of the pulp. This may or may not be accompanied by symptoms [29].
As caries progresses and the inflammatory process builds, the pulp’s unique hard-tissue encasement has a role in ongoing tissue damage [29]. The repair of damaged pulp tissue is also affected by the limited capacity for drainage, and thin-walled vessels that are prone to collapse as intra-pulpal pressure builds. Intra-pulpal pressure increases as a consequence of inflammatory edema. As circulation shuts down, local areas of pulpal ischemia may expand quickly or over an extended period.
Radiographs provide little or no insight into the dynamics of the pulp’s reaction to caries. However, a radiograph may reveal calcification of a chamber adjacent to a long-standing carious lesion. This reaction represents a defensive reaction of the pulp to an irritant that may ultimately complicate endodontic access. Similarly, long-term low-level inflammation may cause diffuse calcification in the root canal system that can impede endodontic instrumentation. Long-term inflammation can also sensitize nociceptors (pain receptors) and complicate local anesthesia (Fig. 5.1).
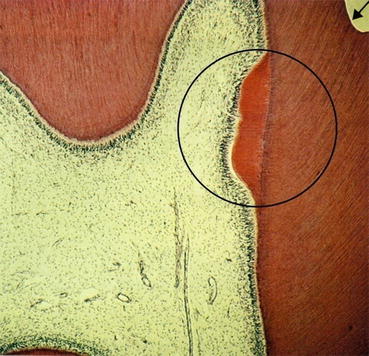

Fig. 5.1
Within the circled area, repair of dentin has occurred in response to a previous carious lesion. In the upper right-hand corner, the arrow indicates the result of a restoration
5.2.1 Host–Microbe Interactions in the Root Canal System
Multiple complex reactions involving attempts at repair and tissue breakdown may go on either asymptomatically or with pain ranging from mild to unbearable. Pulpitis, due to caries, has been described as an infection where the host reaction has the capacity to produce more damage than that caused simply by the effects of the microorganisms [29].
The outcome of the carious process, with or without pulp exposure, is subject to many variables. There are genetic factors involved in host response that are only now being elucidated. Age of the patient also plays a role in the capacity of tissue to respond to an insult. Prior dental experiences are also a factor. A tooth with periodontal disease and/or deep restorations often has a chronically inflamed pulp. Such a tooth is less likely to respond favorably to additional insults than a virgin tooth with healthy pulp and periodontal tissues. The clinician and his/her skills is also an important variable.
Pain, due to caries, is influenced by release of microbial metabolic products and growth factors from the dentin extracellular matrix. At the same time, the tubular characteristics of dentin and the extent of tubular sclerosis due to the reactionary response of the pulp-dentin complex affect the permeability of dentin. The degree of permeability affects the diffusion kinetics of the metabolic and degradation products into the pulp [31].
Response to Caries
There are vascular and cellular reactions as bacteria and toxins in the dentinal tubules approach the pulp. Increased vasodilation is caused by the release of vasoactive mediators including histamine. The initial cellular response of the pulp to carious exposure includes the infiltration of polymorphonuclear neutrophils (PMNs) and monocytes. Acute inflammation and tissue destruction follows, beginning with the formation of microabscesses and necrotic foci in the pulp, and eventually results in total pulp necrosis.
The process involves complex inflammatory and immune responses involving mediators and inflammatory cells. The periapical response to bacterial toxins and mediator release ultimately results in loss of bone and granuloma, periapical cyst, or abscess formation.
Pain is initiated by tissue damage that results in the release of chemical mediators. The mediators stimulate vasodilation and a cascade of reactions, which can result in formation of prostaglandins and other pain inducing mediators. Nonsteroidal anti-inflammatory medication (NSAIDs) can be used preemptively to block formation of pain inducing factors, such as prostaglandins. Please see Chap. 8 for a discussion of NSAIDs.
5.3 Low-Compliance System and Pain
The unique anatomy of the root canal system is an important complicating factor in patients’ painful response to pulp damage. Elsewhere in the body (with the exception of the brain/cranium) as inflammation progresses fluid (edema) accumulates and causes swelling. “Unyielding walls of dentin” [48] is a descriptive phrase with a long history. It describes the fact that dentinal walls encasing the pulp do not allow for tissue expansion and prevents swelling. The relationship between the pulp and surrounding dentin walls has been characterized as a “low-compliance system.” Due to the inability of the pulp to swell, inflammatory fluid accumulates in the confined space of the root canal, ultimately resulting in an increase of intra-pulpal pressure usually associated with pain.
Depending on its specific protein content, inflammatory fluid is termed a transudate, exudate, or pus. Pus is associated with an infectious process and contains variable amounts of dead polymorphonuclear leukocytes, bacterial elements, and blood.
Vasculature of the pulp is characterized by thin-walled vessels with rich collateralization and arterial–venous shunting [23]. As fluid accumulates in the canal system, and tissue pressure increases, areas of arterial vasculature collapse. Ultimately venous drainage becomes overwhelmed and shuts down. Areas of the pulp deprived of circulation become ischemic and ultimately necrotic.
Clinical Tips
-
The low-compliance system is a clinically meaningful concept. Endodontic pain is often associated with increased intra-pulpal or periapical tissue pressure.
-
Emergency treatment: pulpotomy, extirpation of the pulp, and incision and drainage are all directed at reducing intra-pulpal and/or periapical tissue pressure as a means of eliminating pain.
5.3.1 Necrotic Pulp
Necrotic pulp is a dead tissue without functioning vasculature or structural integrity. It provides an ideal culture medium for bacterial colonization. In some cases this process unfolds rapidly and in others very slowly. Pain associated with the process is unpredictable and ranges from virtually intolerable to painless. Clinicians may see large carious exposures of long duration that have caused no pain in contrast to small carious pulp exposures that are extremely painful.
Histologically, it is common to find, within the same pulp, areas of acute inflammation, chronic inflammation, and necrosis. This is a dynamic process and the exposed pulp should be considered a tissue with numerous outcome possibilities. It may proceed to necrosis with or without symptoms or remain as an asymptomatic or symptomatic vital tissue for an indeterminate period of time. A better endodontic outcome is likely if pulp pathosis is treated before periapical pathosis develops.
Sensibility tests of a tooth with a carious exposure should be considered within the context of a dynamic tissue undergoing change. It is well established that pain and sensibility testing are not predictors of the histologic state of the pulp. Despite a vital response of a tooth to pulp testing, it should not necessarily be considered a normal pulp. Exposures and traumatized teeth with partial necrosis may have enough viable neural tissue remaining, to respond to provocation, and mislead a clinician. Ultimately, bacteria in the root canal system results in pulp necrosis.
5.3.2 A Classic Study
A classic study demonstrated the critical role of bacteria following a pulp exposure and the tissue’s reaction to the presence of bacteria. Using a rat model, it was found that in the presence of bacteria, pulp tissue became partially necrotic within 8 days and completely necrotic with formation of periapical abscesses by 14 days. This response was not seen in germ-free animals with pulpal exposures.
At 32 days after pulp exposure in germ-free rats, an intact dentin bridge developed with normal pulp tissue beneath the newly formed dentin. Thus, bacterial infection of the pulp is the key etiologic factor for pulp necrosis. Endodontic therapy must include materials and methods that significantly reduce or eliminate bacteria [22].
This classic study is central to our understanding of endodontic success and failure.
Clinical procedures must be judged in the context of their ability to diminish or eliminate bacteria in the root canal system.
5.4 Trauma
Trauma causes a significant number of painful dental emergencies. Unlike the initial chronic inflammatory response to caries, the reaction to trauma may be immediate devitalization or may not become apparent for years. Excellent texts on the subject of trauma provide guidance for the clinician in treating patients with traumatic injuries. The reader may pursue in depth information on dental trauma in Traumatic Dental Injuries [2]. A useful website that links the best available evidence to treatment of traumatic injuries and expected outcomes can be accessed at www.dentaltraumaguide.org/.
Developed by Dr. Jens Ove Andreasen, the site is unique in its accessibility and value to all dentists treating traumatic injuries.
5.4.1 Restorative Factors
Restorative procedures can be the cause of pulpal problems. An important issue is microbial microleakage [41]. Penetration of microbes into the deeper sections of dentin and pulp can cause severe pain that may be difficult to diagnose. The durability of bonding and clinical success of adhesive restorations is an important area for continued research [9]. Iatrogenic factors during restorative procedures such as inadequate coolant and generation of heat are also potential causes of pulpal breakdown.
It is common to have a patient complain of thermal sensitivity following a restorative procedure. A key question for the clinician involves determination of whether or not pulpitis and resulting thermal sensitivity is reversible or irreversible. The patient’s level of pain and its duration are subjective findings but are important factors in judging the probability of reversibility or irreversibility of the process.
Clinical Tips
-
Sensitivity to percussion, if hyper-occlusion can be ruled out, is a critical sign indicating that pulpal inflammation has extended from the pulp into the periodontal ligament. This biological event usually signals an irreversible pulpitis.
The aim of the wise is not to secure pleasure but to avoid pain Socrates
5.5 Pain-Preventive Strategies
5.5.1 Preoperative Strategy
Biologically based preoperative, intraoperative, and postoperative strategies can have a preventive effect on patient’s pain and endodontic experience. A pain-preventive or preemptive strategy is a multifaceted plan directed at prevention or reduction of intraoperative and postoperative pain. The strategy includes anxiety reduction, profound local anesthesia, biologically based operative and postoperative procedures, and preemptive use of analgesics.
Clinical Tips
-
Pain is more difficult to eliminate after it has been established.
-
A pain-preventive strategy is based on a preemptive approach using appropriate local anesthetics (longer lasting), preemptive analgesia, anxiety reduction techniques, and clinical strategies.
5.5.2 Pain: Predisposing Factors
Most clinicians have treated two patients of the same gender with similar teeth and medical/dental histories. The teeth are treated using the same clinical approach but one patient has a smooth postoperative course while another experiences complications including severe pain and swelling. The clinician often looks for an iatrogenic cause of the pain. While iatrogenic factors often exist, there is increasing evidence that there are other factors that may predispose some patients to complications.
This section will examine the potential role of predisposing factors and comorbidities including genetics, gender, and anxiety.
5.5.3 Comorbidities
A comorbidity has been defined as a concomitant but unrelated pathologic or disease process [1].
For example, it has been suggested that patients with chronic pain conditions, elsewhere in the body, may be predisposed to prolonged pain after apparently successful endodontic therapy [40]. Patients with a high level of anxiety or chronic facial pain problems are examples of comorbidities that may affect the patient’s experience during and after endodontic therapy. A patient’s sex may also be a factor as there is evidence that females may be predisposed to certain painful conditions.
5.5.4 Predictors of Endodontic Pain
Although endodontic treatment can be virtually pain free during the procedure itself, some patients may experience varying degrees of pain following treatment. It is helpful for the clinician to recognize factors that are predictive of postoperative pain. That information may influence the endodontic treatment plan. For example, a decision concerning single or multi-visit treatment could be influenced by knowledge of predisposing factors [20].
Studies have investigated postoperative endodontic pain and reported an incidence of moderate to severe pain in the range of 15–25 % [10, 21, 37]. A prospective clinical study reported that 57 % of patients reported no pain after debridement and shaping of the root canal system, although 21 % had slight pain, 15 % had moderate pain, and 7 % had severe pain [17].
Although some patients may experience moderate to severe pain after endodontic treatment, few experience what is now commonly referred to as a flare-up or a postoperative problem requiring an unscheduled visit with unplanned treatment intervention to manage the patient’s symptoms [50]. Patients with a flare-up usually describe severe pain, swelling, or the sensation of pressure in their mandible or maxilla within 1 or 2 days of treatment.
The incidence of flare-up varies across studies and ranges from about 2 to 20 % of patients, with the higher prevalence generally reported in older studies using classic cleaning and shaping techniques [5, 46, 47]. Differences in experimental design prevent direct comparison of those research results, but the presence of preoperative pain or mechanical allodynia (reduced mechanical pain threshold or percussion sensitivity) was a positive predictor of postoperative pain in more than 15 studies involving more than 6,600 patients.
Other factors were more variable in their predictive value of postoperative pain. In a retrospective study, the dental records of 1,000 patients who had nonsurgical root canal treatment and experienced no flare-ups (i.e., unscheduled return visits) were compared with the records of 1,000 patients who experienced flare-ups after the cleansing and shaping of their necrotic root canals [45]. The results showed that factors such as presence of preoperative pain, tooth type, sex, age, history of allergy, and retreatment were significantly predictive for the incidence of flare-up, although intra-canal medicaments, systemic disease, and establishment of patency of the apical foramen had no significant relation to the incidence of flare-ups. Specifically, the highest incidence of flare-ups was associated with mandibular teeth, retreatment procedures, females over the age of 40, and patients with a history of allergies [45].
5.6 The Influence of Other Factors on Patients Pain: Genetics, Sex, and Anxiety
Based on the best available evidence, it seems likely that there are factors that predispose a patient to pain. These factors may alter the patient’s pain threshold as well as their response to tissue injury and treatment. Some of the factors are apparent such as the patient’s sex, while others like the patient’s genetic background remain unknown to clinicians. This is likely to change in the future (Figs. 5.2 and 5.3).
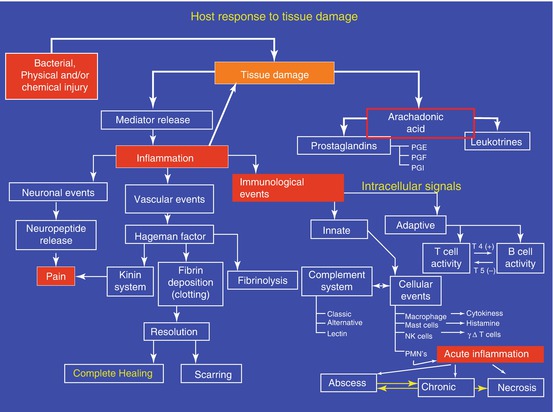

Fig. 5.2
Dynamic response of a patient to tissue damage

Fig. 5.3
Comorbidities can be complicating factors during endodontic treatment
The subject of predisposing factors is undergoing scrutiny, and it is reasonable to assume that there will be further elucidation of important predictors of pain. It is interesting to consider the future and the implications of increasing knowledge in the area of genetics and the role the patient’s sex may have on our prevention and treatment of pain.
5.6.1 Genetics
The role of genetics is an entirely new variable for dentists to consider. Genetics may play a role in predisposing some patients to a variety of complications including pain, poor healing, and abscess formation. Genetic factors may also be important in determining how a person responds to specific drugs. Considering the role of genetics in endodontics is highly complex and at an early stage of development.
Increasingly, current endodontic literature provides examples of how genetics may influence endodontic symptoms and outcomes. Findings suggest that specific markers associated with the pro-inflammatory regulator Il-1B, a key regulator of host response, may contribute to increased susceptibility to periapical pathosis [32]. It has also been suggested that genetic factors are associated with a susceptibility to develop symptomatic dental abscesses [11].
Numerous genes are involved with the pharmacokinetics and dynamics of opioids thus complicating the issue of genetics and patient’s response to an opioid. A variety of genetic polymorphisms clearly influence pain perception and behavior in response to pain. The response to analgesics differs depending on complex factors including the pain modality and the potential for repeated noxious stimuli, the opioid prescribed, and even its route of administration [49].
The Human Genome Project has contributed to the possibility of the development of drugs specific for individualized therapy. It seems clear that genetic variations influence both the efficacy and side effects of drugs used to treat pain. A study examined genetic and environmental contributions to variability in pain sensitivity and responsiveness to opioid analgesics. Findings indicated that interindividual variance in responsiveness to opioids is at least in part due to genetics [3]. More than 20 genes affecting pain sensitivity in humans or interindividual variability have been identified. While at this time some of the data is conflicting, it is exciting to think about what the future of genetics may mean to endodontists of the future [33].
For example, it has been suggested that markers in MMP3 and MMP2 genes could predict host susceptibility to developing periapical lesions and the healing response. Genetic predisposition in specific genes can contribute to persistent apical periodontitis [30]. It seems likely that an understanding of the genetic basis of endodontic pain perception will advance our pharmacologic management of postoperative pain.
These preliminary findings point to a rich complex of factors associated with patient’s pain and treatment outcomes. It is possible that as more information is collected, we will be better able to identify those patients predisposed to pain and have a diminished capacity for healing.
5.6.2 Sex and Gender
Terminology
Although biological sex exerts a major influence on a person’s gender identity, “sex” and “gender” are not interchangeable terms. The term “sex” refers to biologically based differences, while the term “gender” refers to socially based phenomena. If research subjects are to be categorized by anatomic features (chromosomes, reproductive organs), it is appropriate to describe the study as one of “sex differences.” In contrast, if additional measures of masculinity/femininity or gender identity are used to describe subjects, then the term “gender differences” is appropriate [19].
Pain Responses: Men and Women
During the last 10–15 years, there is a growing body of evidence indicating that there are substantial sex differences in clinical and experimental pain responses for women and men [15]. It seems that women are at a substantially greater risk for many clinical pain conditions. An extensive review reported that a survey of the currently available epidemiological and laboratory data indicates that there is overwhelming evidence for clinical and experimental sex differences in pain. Numerous reasons for these findings have been given, including hormonal and genetically driven sex differences in brain neurochemistry [49]. Furthermore, some highly prevalent chronic pain syndromes that are found in both sexes (including chronic fatigue syndrome, fibromyalgia, interstitial cystitis, and temporomandibular disorder) occur overwhelmingly more often (in more than 80 % of cases in which treatment is sought) in women.
Interestingly, it was observed that natural redheaded women required 19 % more desflurane (volatile anesthetic) than women with dark hair. Initially this observation was reported anecdotally, but the observation was investigated and it was determined that red hair in women was the result of a genetic variant and also a distinct phenotype associated with anesthetic requirements in humans [26]. In 2005, it was found that there was increased thermal sensitivity and reduced subcutaneous lidocaine efficacy in redheads. However, another study was unable to replicate those findings when inferior alveolar nerve blocks were evaluated [12].
5.7 Recent Research: Probability of Pain
A clinical study investigated the probability of the incidence, intensity, duration, and triggering of post-endodontic pain, considering factors related to the patient (age, gender, medical evaluation) and the affected tooth (location, number of canals, pulp vitality, preoperative pain, periapical radiolucencies, previous emergency access, and presence of occlusal contacts) [4].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


