(14.1)
The calcium to phosphate molar ratio can be adjusted accordingly to yield different forms of calcium phosphates. In certain circumstances of drug delivery applications, tricalcium phosphates (TCP) present the more ideal composition compared with other calcium phosphates. TCP has been extensively studied for use as bone grafts [25–28] and for drug delivery systems [29–32] owing to appropriate dissolution rate [33–35]. The hydrothermal conversion from calcium carbonate exoskeletons to TCP would require a Ca/P = 1.5. The time variant is an important factor as <24 h conversion would yield carbonated TCP while 48 h would complete the transformation [14]. Obviously this also depends on the size of the material converted.
Chemical compositional analysis of marine structures can be examined by various techniques including mass spectroscopy. Elemental quantification by inductively coupled plasma-mass spectroscopy (ICP) (Table 14.1) exhibits that the majority of the calcium, magnesium and strontium ions are preserved during this conversion process.
Table 14.1
Ionic composition of Foraminifera before and after hydrothermal conversion to β-TCP
|
Calcium
|
Phosphate
|
Strontium
|
Magnesium
|
|
|---|---|---|---|---|
|
(mg/g)
|
(mg/g)
|
(mg/g)
|
(mg/g)
|
|
|
Before conversion
|
17 ± 0.5
|
0
|
1.8 ± 0.05
|
30.6 ± 0.4
|
|
After conversion
|
17 ± 0.7
|
62 ± 9
|
2.1 ± 0.07
|
30.7 ± 0.2
|
14.3 Drug Delivery Systems Based on Calcareous-Derived Calcium Phosphates
The therapeutic efficacy of any drug delivery system is dependent on how the drugs are loaded, how efficient are the drug loading and, most importantly, the release rate of the loaded drugs. Marine calcareous materials provide the ideal internal porous structure for drug loading, which this chapter will further discuss.
A key benefit in employing the use of hydrothermal exchange is the preservation of the structural integrity of the original material. The chemical composition has changed, but the structure remains intact as shown in Fig. 14.1. There exist a variety of strategies that can allow incorporation of pharmaceutics in this type of carrier material as illustrated in Fig. 14.2.
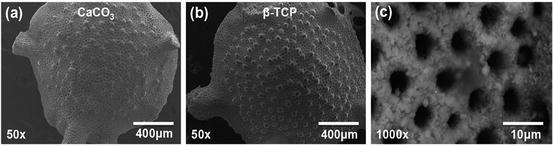
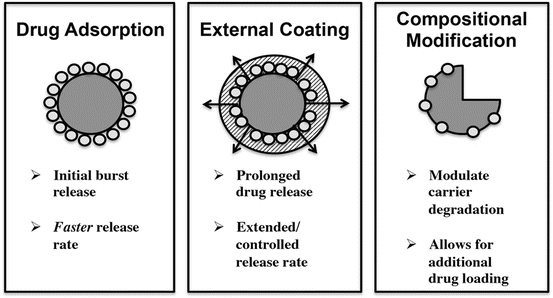

Fig. 14.1
Scanning electron micrographs showing (a) Foraminifera (CaCO3) before conversion and (b) after hydrothermal conversion to β-TCP. The surface pores (c) are uniformly distributed and are approximately 5 μm in diameter

Fig. 14.2
Schematic summary detailing the strategies available for drug loading on carrier material such as calcareous-derived calcium phosphates
The most common and simplistic method of drug loading is through immersion of the carrier material in a concentrated solution containing the pharmaceutics for a period of time. These drugs are in turn adsorbed onto the surface and within the material, which will depend on the porosity of the carrier. The interconnected and uniformly porous network of the calcareous exoskeleton can provide a consistent and reliable drug loading as opposed to irregular porous materials. Once the system is placed in its intended environment, drugs will begin to be released into the surrounding environment initially through the surface of the carrier and as the pores are infiltrated through diffusion mechanism.
This characteristic can be observed by plotting a drug release plot based on the Higuchi Eq. (14.2), which the linearity of the graph will determine if the release is by diffusion through the matrix material:
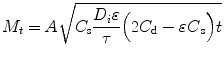
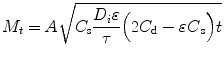
(14.2)
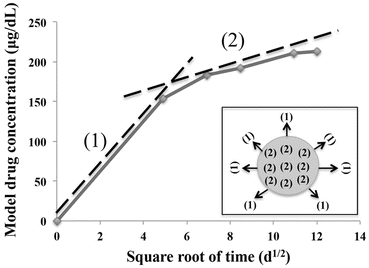
where M t is the amount of drug release after time t, A is the matrix surface area, D is the diffusion coefficient of the drug, C s is the solubility, C d is the concentration of the drug in the matrix, τ is the tortuosity and ε is the porosity of the matrix. The release mechanism was demonstrated using a model drug incorporated with the calcareous-derived calcium phosphate material and plotting a subsequent drug release profile based on the Higuchi plot shown in Fig. 14.3.

Fig. 14.3
Higuchi plot showing two phases of drug release: surface release slope (1) and drug diffusion release from the matrix material slope (2)
The plot shows two phases of the drug released. The initial burst release was due to conventional release of drug from the surface of the carrier material as illustrated by slope (1). The second-order release profile shows a linear slope (2) in the Higuchi plot suggesting that the release of the drug from the matrix material is based on a drug diffusion process through the macro- and micropores of carrier. This provides an overview on the release mechanism profile from using these carrier materials and can allow further optimisation by controlling these release profiles.
14.3.1 Controlled Release of Bone Stimulatory Drug: Simvastatin
Bone repair and formation is a complex process that would require stimulatory compounds in the form of pharmaceutics, growth factors, proteins, etc., to assist in the regeneration process. In the case of osteoporosis, where there is an imbalance in bone remodelling process, the use of stimulants is even more crucial. The last few decades have witnessed the development of various bone stimulatory drugs like bisphosphonates and its derivatives and more recently simvastatin. In previous studies, simvastatin was successfully loaded with the Foraminifera-derived β-TCP (SV/β-TCP) with a 75 % loading efficiency. To control the release of simvastatin and control its release rate, an apatite coating was made around the β-TCP material (Ap/Sv/β-TCP) [36]. This reduced the release of simvastatin from 44 % down to 22 % which gave an approximately 50 % reduction in the release (Fig. 14.4).
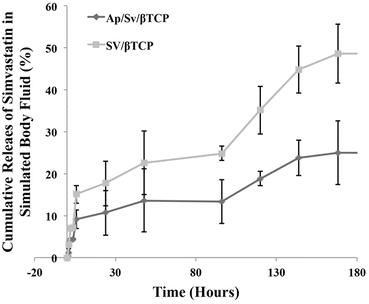

Fig. 14.4
The release of simvastatin in simulated body fluid over 7 days from β-TCP (SV/β-TCP) and apatite-coated β-TCP with simvastatin (Ap/Sv/β-TCP) showing a reduction of simvastatin release equal to approximately 50 %
This will allow a more prolong release of the drug into the local area thereby increasing the therapeutic efficacy of the system. This system was tested in an osteoporotic mice model where significant cortical and cancellous bone formation was observed in the localised area [37]. Furthermore, Ap/Sv/β-TCP produced significantly stronger bones compared with the experimental groups. This is thought to be the effect of slower local release of simvastatin, which again reinforces the potential benefits of using this drug delivery system. However, if the treatment is aimed for systemic applications such as osteoporosis, new strategies are needed to be developed. To assess the difference between local and systemic delivery in a separate study, Ap/Sv/β-TCP system was compared with direct injection of equal amount of simvastatin. The results over the 6-week experimental period showed that direct injection ignited severe localised muscle inflammation whereas the Ap/Sv/β-TCP showed no sign of any adverse effects [38].
This is again attributed to the slower controlled release of simvastatin within the range of therapeutic efficacy compared with direct injection of the drug. Depending on the application and the duration of the therapy required, the unique structures of Foraminifera could be adapted and modified to achieve the desired therapeutic effect.
14.3.2 Carrier for Antibiotic (Gentamicin) Against Methicillin-Resistant Staphylococcus aureus (MRSA)
Despite advances in hygiene management in surgical protocols, bacterial infections are still prevalent in modern surgeries. In second- or third-world operating theatres, the cases for infections are even higher. In orthopaedic surgery, Staphylococcus aureus (S.aureus) are the most common strain in the cause of infections. These infections generally go unnoticed till it is too late as the bacteria progressive proliferate and spread in the body. With bacteria evolving into superbugs and becoming ever more resistant to antibiotics, the use and application of antibiotics is even more crucial especially in the hospitals. PMMA loaded with antibiotics are widely used in orthopaedic surgeries as the treatment of choice. PMMA is not biodegradable and as such would require another surgery to remove it after its intended function. This would again risk the patient the chance of repeated infection. What is agreed upon scientists is the need for effective treatment and prevention systems against S.aureus and its kind ideally by a biodegradable carrier. With this aim in mind, foramina-derived β-TCP was loaded with gentamicin-sulphate antibiotic (GS-TCP) and evaluated to treat and/or prevent the occurrence of clinical strain methicillin-resistant Staphylococcus aureus (MRSA) in vitro [39]. The study showed that a single GS-TCP was capable of releasing antibiotics to prevent the growth of MRSA during its exponential growth phase.
Furthermore, a time-delayed study where GS-TCP was introduced to the MRSA at various times (5, 10, 15, 30, 60 and 1,440 (24 h) min) showed the negative presence of the MRSA thereby signifying the potential antibacterial efficacy of the GS-TCP (Fig. 14.5). Though this would still require further in vivo investigations and optimisation before it can be applied in a clinical setting, the results suggest that the carrier material can release antibiotics at a level that is therapeutically relevant in the prevention and treatment of MRSA and can be applied solely as an antibiotic treatment or implanted as a bone filler with antibiotic potential.
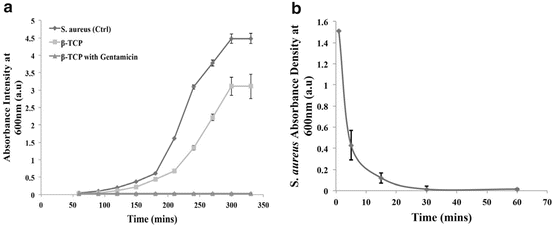

Fig. 14.5
Introducing the antibiotic (gentamicin)-loaded β-TCP Foraminifera to MRSA shows (a) inhibition on the growth of MRSA, and (b) time-delay introduction of the system shows prevention of MRSA growth after 30 min
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


