Fig. 10.1
Typical cauliflower morphology of hydroxyapatite when deposited on the surface of a bioactive biomaterial (Reprinted from Ref. [20], Copyright 2007, with permission from Elsevier)
In a bone tissue engineering perspective, bioactivity can be categorized into class A, when the biomaterial presents osteoproduction (bone growth in the bulk of the biomaterial) and osteoconduction (stimulation of bone growth at the interface), and class B, when it only presents osteoconduction [18].
In vitro bioactivity test evaluates the ability of the biomaterial to form an apatite surface layer when in contact with simulated body fluid (SBF), a solution that presents ionic concentrations similar to human blood plasma [21, 22].
After the initial description of this methodology, a series of modifications have been proposed in the composition of SBF. One of the most relevant is mSBF that presents ionic concentrations equal to the human plasma, with the exception of HCO3 –, whose concentration was reduced to the level of saturation of calcite [23]. This type of approach is also used as a surface modification methodology, generating an apatite coating that enhances the bioactivity of the biomaterial [24]. Other modifications to the SBF solution have been reported, the use of higher ion concentrations (e.g., 1.5 × SBF, 5.0 × SBF, among many others) to reduce the time frame of the apatite deposition are common [24, 25].
The use of SBF or SBF-like solutions is one of most commonly used approaches to test the bioactivity of a biomaterial. However, the different steps of this methodology should be carefully controlled, and the conclusions that one can take from such a characterization are not consensual [19, 26].
10.3 Synthesis and Preparation of Bioactive Composite Systems
10.3.1 Bioactive Inorganic Particles and Their Synthesis
Bioactive inorganic particles (glasses or glass–ceramics) are usually composed of silicates or phosphosilicates (as network formers) combined with different proportions of glass modifiers, e.g., sodium oxide (Na2O) and calcium oxide (CaO), among others. They have been studied for more than 40 years and are characterized by their bioactivity and unique bone bonding properties, which are usually related to their surface chemistry. A landmark on the development of these systems was the work of Hench et al. [27]. They showed that the bioglass 45S5 (with a general composition 0.461SiO2:0.026P2O5:0.269CaO:0.244Na2O) is able to promote the formation of a calcium phosphate layer on its surface within a time frame of 30 days. This glass composition is one of the most studied ones [27–29]. The concentration of SiO2 in the glass/glass–ceramic structure seems to be a critical parameter that governs the bonding of the material to living tissue [27]. It has been proven that glass formulations that present fast bonding not only to bone but also to soft tissues are prepared with 45–52 % (by weight) of SiO2 [30]. Glasses with a SiO2 content ranging from 55 to 60 % react more slowly and do not bind to soft tissues. Compositions with >60 % of SiO2 do not bond to bone and are considered as bioinert [27, 31]. Additionally, bioactive glasses have been demonstrated to stimulate the growth and maturation of osteoblasts [31–33]. Most of these materials degrade naturally, similarly to other synthetic substitutes, such as calcium phosphates (CaPs). The degradation products are, usually, metabolized by the body and excreted through the urine [34].
The brittle behavior and weak mechanical properties observed in bioactive glasses are the major difficulties associated with their use in biomedical applications. The bending strength of most bioactive glasses is in the range of 40–60 MPa, which is not enough for load-bearing applications, while their modulus is between 30 and 35 GPa, very close to that of cortical bone. The combination of biodegradable polymers with bioactive glasses has been proposed to produce biodegradable products that show high levels of bioactivity and also improve some of their mechanical properties as compared to conventional glasses [35–37].
Bioactive glasses and glass–ceramics can be obtained by melting at high temperatures followed by casting into a mold or splat quenching. An alternative approach for glass synthesis is the sol–gel route [29, 38, 39]. Both strategies are detailed in the following sections.
10.3.1.1 Melt-Based Approach
Bioactive inorganic particles can be synthesized by different methodologies, and the melt-quenching approach is one of the most traditional and straightforward routes. Under this procedure, the glass precursors (e.g., silica, phosphates, carbonates, etc.) are mixed in a mortar, transferred to a crucible, and fired to temperatures able to melt the whole mixture that can go up to 1,600 °C or higher, depending on the formulations [16, 40–44]. The homogeneous mixture is then splat quenched into ice water or onto a metal plate maintained at room temperature. This process allows the chemical structure to be frozen [13, 45]. Depending on the starting materials, the heating process can be done stepwise, namely, 300 °C if the starting materials contain ammonia in their chemical structure (e.g., sodium ammonium hydrogenophosphate) and/or 650 °C if the starting materials are carbonates (e.g., calcium carbonate). These thermal treatments allow the release of by-products, such as ammonia or CO2 that diffuse through the precursors and are released from the mixture [40–42]. Bioactive glasses prepared by melting are usually denser and do not contain any remnants of organic components or water; nevertheless, melt-derived bioactive glasses have a limited surface area, being one of their main disadvantages when in contact with the biological tissues or fluids [46, 47]. The high temperature used in the fabrication of these inorganic particles through the conventional melt-based process does not allow the incorporation of bioactive organic molecules/materials (e.g., proteins, growth factors, genes, hormones, etc.) during the fabrication process.
10.3.1.2 Sol–Gel Approach
Advances in sol–gel processing technology allowed the manufacture of a new generation of bioactive glasses overcoming some of the drawbacks of the melt-based approach (e.g., high processing temperatures) [48]. This low-temperature process dispenses the use of Na2O in bioactive glass compositions (the main role of this component in the glass compositions is related to the lowering of the glass melting temperature) and allows incorporating polymers and organic molecules to make less brittle hybrid materials [11]. Also, sol–gel route allows a wider range of bioactive compositions for a better response to specific clinical applications, permits the easier control of its morphology and chemical composition, enables an easier design of the material’s morphology (powders, monoliths, nanoparticles, gels), and generates materials with high specific surface area, osteoconduction properties, degradability, and nanoporosity [35, 45–47, 49]. In fact, the main physical differences between melt and sol–gel-derived glasses are that the latter ones tend to have inherent nanoporosity, whereas melt-based glasses are denser. The nanoporosity can result in improved cellular response due to the nanotopography that might reach a surface area 100× higher than for similar compositions produced through the melt-based approach [50, 51]. This property increases the solubility of the glass, which is important in a bioactivity perspective [11]. This characteristic is confirmed by in vitro (in SBF) and in vivo studies that report a higher bioactivity and degradability of the sol–gel-derived glasses when compared to the melt-derived ones [51].
The sol–gel route essentially forms and assembles nanoparticles of silica at room temperature. It is a synthetic route where a solution containing the glass compositional precursors (e.g., alkoxides or metal chlorides) undergoes hydrolysis and condensation reactions to form a gel either in water or in an organic solvent. A typical silicate precursor is tetraethyl orthosilicate (TEOS), while common precursors for calcium and phosphate groups are calcium nitrate tetrahydrate and triethyl phosphate, respectively [11, 52]. Adding water or a water/alcohol mixture to TEOS promotes the hydrolysis of the alkoxide functionality generating silicic acid. The addition of an acid or a base as a catalyst allows the silicic acid to condense into a network of silica-based gel through the release of water molecules. Depending on the experimental conditions and compositions, these two steps might occur simultaneously [53]. At the initial time frame of the silicic acid condensation, a sol is generated. The subsequent agglomeration of the particles that constitute the sol forms the gel. This gelation step proceeds slowly. Complete cross-linking of the silicate network is achieved during the ageing step [53, 54]. A procedure can be adapted to generate glass particles and not a 3D glass structure (e.g., Fig. 10.2). In this approach the experimental conditions are manipulated to inhibit the agglomeration of the particles generated in the initial steps of the procedure [55, 56]. Typical bioactive compositions can be binary systems, e.g., SiO2-CaO; ternary systems, e.g., SiO2-CaO-P2O5; or quaternary systems, e.g., SiO2-CaO-Na2O-P2O5 [52, 57, 58]. The silanol group is critical for the bioactivity of the glass. In addition, gel-derived bioactive glasses may contain high-energy silicate ring structures, which further activate the material reactivity [12, 14, 15].
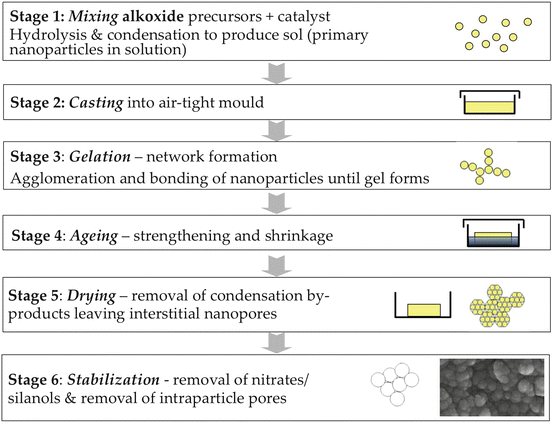

Fig. 10.2
A flow chart of the acid-catalyzed sol–gel process used to synthesize bioactive glasses, including schematics of the evolution of the gel and its nanoporosity (Reprinted from Ref. [11], Copyright 2013, with permission from Elsevier)
Microparticles, monoliths, or foams are usually produced using acidic catalysis (Fig. 10.2) [11]. Using this methodology, the primary nanoparticles (with diameters around 2 nm) present in the sol coalesce and condensation (polymerization) occur, forming Si–O–Si bonds. The nanoparticles coarsen, coalesce, and bond together, forming a gel network of assembled nanoparticles [59]. The gel is dried and is heated at temperatures above 700 °C to produce a nanoporous bioactive glass [11]. As water and alcohol evaporate during drying, they generate an interconnected porous network. The pores are formed in the interstices between the coalesced nanoparticles [59], and their size depends on the precursors used, the glass composition, and the pH of the reaction [60]. Pore diameters are typically in the range of 1–30 nm [52].
If the synthesis is carried out under basic conditions, submicrometer particles are formed [61]. Compared with micron-sized bioactive ceramic particles, nano-sized particles have a higher specific surface area and can form a tighter interface with the polymer matrix in a composite formulation [62–65]. Furthermore, reducing the size of the particles would not only accelerate the formation of a bioactive hydroxyapatite surface layer but also provide more active sites for osteoblast attachment, enhancing their proliferation and differentiation, as well as tissue growth [66, 67]. Also, they exhibit, to some extent, similar nanoarchitecture as the physiological bone [68]. Work on the fabrication of bioactive glass nanoparticles (BG-NPs) by the sol–gel route has been reported [55, 65, 69]. Hong et al. obtained ternary BG-NPs (SiO2-CaO-P2O5) by the combination of two strategies, sol–gel and coprecipitation approaches. The mixture of precursors was hydrolyzed in an acidic environment and condensed in alkaline condition, and the resultant particles were collected by freeze-drying [62]. Briefly, the sol–gel synthesis procedure comprised as follows: mixture of TEOS, Ca(NO3)2, ethanol, and water; addition of citric acid (catalyst) to adjust solution pH at 1–2; vigorous agitation to promote the hydrolysis of the silica precursor; adding drop by drop the resultant sol into a (NH4)2HPO4 solution under vigorous agitation; continuously adding ammonia into the solution to maintain the pH at 10–11; separating the particles by centrifugation; and the collection after freeze-drying. After calcination at 700 °C, it was possible to obtain particles with an average diameter of around 20–40 nm [62]. It was found that both binary and ternary BG-NPs prepared by this method exhibit bioactive features [70]. Formulations, morphologies, and sizes of BG-NPs could be tailored by varying the production conditions and the feeding ratio of the reagents [56, 71]. For instance, Chen et al. investigated the effects of the experimental conditions on the morphology of BG-NPs of the system SiO2–CaO–P2O5, and they found that the use of lactic acid decreased the size of the BG-NPs [72].
The sol–gel versatility allows the incorporation of different ions to the glass structure (e.g., Zn2+, Mg2+, Ag+, etc.) in order to improve the glass functionality and bioactivity [43]. El-Kady et al. produced silver-doped BG-NPs that showed antibacterial activity against different types of bacteria. This type of particles could be used to minimize the occurrence of bacterial infections generated by the implantation of bone tissue engineering scaffolds [73].
The sol–gel technique has been also applied in the synthesis of a variety of bioactive monolithic and particulate glasses (e.g., Fig. 10.3). However, a high-temperature calcination step is required to eliminate organic remnants, the processing is relatively time consuming, and it is difficult to obtain defects-free bioactive glass monoliths with diameters above 1 cm. Defects are mainly due to the shrinkage that occurs during drying. For particles, vapor stresses through the interconnected pore network are small, and the path of evaporation is short, although, for monolithic objects, the path from the center of the monolith to the surface is long, and the drying stresses can introduce fracture [11, 74]. The enumerated drawbacks of the sol–gel route, allied with the low cost of the melting procedure makes this latter route dominant in the commercial production of bioactive glasses [75].
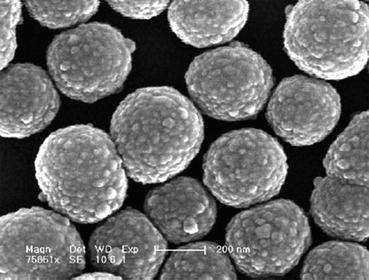

Fig. 10.3
Bioactive silica-based particles prepared through the sol–gel route (Reproduced from Ref. [76] by permission of John Wiley & Sons Ltd.)
10.3.2 Natural-Based Polymeric Phases/Composites and Their Processing
The combination of bioactive inorganic particles with natural-based polymers generated a family of bioactive composites with a wide range of applications, from structural implants to tissue engineering scaffolds [74, 77]. These composites combine the flexibility of polymers with the stiffness, strength, and bioactive character of the inorganic glass fillers. So far, most of the published work on this class of composites has been carried out using conventional (micron-size) inorganic particles as fillers (or coatings) [77, 78]. However, the capacity to produce nano-sized particles/fibers allowed the development of bionanocomposites through the combination of nano-reinforcements and biodegradable polymers of different origins. These bionanocomposites have been explored, for example, as porous scaffolds for bone tissue engineering applications or membranes with potential applications in the dental field [79–81].
These types of bioactive composite structures can be produced using different methodologies, namely, melt-based ones (which requires the use of a thermoplastic polymer), wet chemistry approaches, or rapid prototyping. These three main processing methodologies will be further detailed in the next sections.
10.3.2.1 Conventional Melt-Based Methodologies
Conventional melt-based processing techniques (e.g., extrusion, injection molding, compression molding, fused deposition molding, etc.) are based on the melting of a particular polymer in order to shape it or mix it with different reinforcements [82]. In this perspective, non-thermoplastic materials are not possible to be processed using these methodologies. Most of the natural-based biodegradable polymers are not thermoplastic, limiting their melt-based processing [83, 84]. Researchers have overpassed this limitation through the combination of natural-based polymers with thermoplastic polymers [85]. Under this approach, different polymeric blends, able to be processed using melt-based technologies, were developed (e.g., blends of starch and synthetic polymers and blends of chitosan and aliphatic polyesters) [86–92].
Thermoplastic polymers or blends have been used as matrices for the production of glass-/glass–ceramic-based composite systems [93–98]. Most of the published work targeted their use in bone tissue engineering. The inorganic phases are introduced to enhance the bioactivity of the material, while some of the mechanical properties are improved [96].
Melt-based processing using blowing agents or compression molding followed by particulate leaching has been used to obtain porous inorganic/organic biodegradable composites [99, 100]. It is also worth to highlight that physical blowing agents, such as supercritical CO2 or water, have been shown to be effective on developing porous glass-/glass–ceramic-based composites for bone tissue engineering applications [101]. In fact, porosity is a critical parameter when complete bone regeneration is targeted. It allows the cell colonization in the bulk of the biomaterial and, subsequently, new bone production.
10.3.2.2 Wet-Spinning Approaches
Wet spinning is a phase inversion technique allowing the production of polymer fibers through an immersion–precipitation process. In the methodology, a solution of polymer is continually spun through a spinneret leading to the formation of a polymeric filament. The spun occurs into a coagulation bath composed of a poor solvent (non-solvent) or a non-solvent–solvent mixture with respect to the polymer being processed. A homogeneous solvated filament, composed of polymer, solvent, and possible additives, solidifies because of polymer desolvation, caused by solvent–non-solvent exchange [102]. The essential feature in wet spinning is the transfer of solvent from the polymer to the coagulating bath. The major drawbacks that limit the rate of wet spinning are usually related with the need for sufficient time for coagulation to occur, the dependence on the rate of solvent diffusion, and the considerable viscous drag of the coagulating baths [102].
Through the assembly of the thin wet-spun fiber meshes, it is possible to fabricate 3D networks of fiber meshes. This methodology imparts flexibility to the manufacturing process, allowing the design of scaffolds with properties that can be adjusted depending on the targeted site (e.g., thicker scaffolds for the long bones or membrane-like scaffolds for the maxilla regions) [103, 104]. It has been reported that the combination of wet-spun chitosan fibers with bioglass enhances the material bioactivity and promotes osteoblast proliferation and ALP activity [105]. Wet-spinning technologies also enable to create hybrid materials with high levels of organization and avoid the thermal degradation of natural origin polymers compared to other techniques, such as the melt spinning [103]. Wet spinning has been used for the production of wet-spun fibers for drug release [106] and polymeric scaffolds for tissue engineering applications [104, 107].
10.3.2.3 Rapid Prototyping
Rapid prototyping is based on an approach that combines computer science and manufacturing technologies. The main advantage of these techniques is their ability to produce complex structures using a computer-aided design (CAD) model [13]. Currently, different rapid prototyping techniques are used for biomedical applications and can be classified as (1) laser-based, (2) nozzle-based, and (3) printer-based systems [108].
Laser-based systems benefit from the photopolymerization pathway as a basis to fabricate cross-linked polymeric scaffolds. This type of processing methodology has been also employed to develop 3D scaffolds constructed solely with bioactive glasses [109]. In this approach a CO2 laser is used to soften or melt the glass particles that are injected to the targeted position by means of a nozzle. The processed particles bind to the substrate at the position where they interact with the laser radiation. These 3D constructs exhibit a higher crystallization degree than their parent glass powder precursors. They maintain their bioactive character after processing, although the rate of apatite formation when immersed in SBF is slower [109].
The main limitation of the nozzle-based techniques is that the resolution is determined by the nozzle size, which makes it difficult to design and fabricate scaffolds in the micron- or nano-size. The well-known processing of (pre)-polymers by pressure exerted on extrusion/dispensing units supports the second category of rapid prototyping systems. Under this approach, the production of bioactive glass–polycaprolactone (PCL) composites is achieved by stereolithography, through the printing of PCL–glass mixtures and cross-linking of methacrylated PCL. The resulting 3D constructs are bioactive, being able to generate an apatite layer upon immersion in SBF. Additionally, the constructs revealed to be non-cytotoxic towards fibroblasts, inducing an increase in cell activity when the bioactive glasses were added up to a concentration of 20 % [110].
The printer-based systems combine powder beds and the deposition of a binder that fuses the particles, or directly depositing material using inkjet technology [108, 111, 112]. It has been used to process biodegradable polymeric scaffolds for tissue engineering applications [13]. This process prints a binder onto a surface in a sequential layered way. The processing parameters such as the speed, flow rate, and drop position can be computer controlled to produce complex 3D scaffolds.
Several recent research works have been reported on the fabrication of 3D scaffolds using highly bioactive mesoporous glasses as starting materials. For example, Yun et al. reported the synthesis of ordered bioactive 3D glass scaffolds exhibiting a hierarchical porosity composition using a combination of sol–gel, double polymers templating, and rapid prototyping techniques [113
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


