16 Autoimmune Disorders, Lymphoproliferation, and Granulomatous Inflammation
Sjögren Syndrome (Autoimmune Sialadenitis, Mikulicz Disease, Chronic Sclerosing Sialadenitis)
Chronic Graft-Versus-Host Disease
Introduction
Autoimmune diseases comprise more than 80 chronic diseases that affect 5%–8% of the (general) population. In general, the diagnostic procedures for autoimmune diseases are unsatisfactory, as they are based on clinical signs and symptoms, many of which are very common and may be easily overlooked and considered as unspecific. With regard to salivary gland conditions, the most obvious disorder is Sjögren syndrome. The clinical correlates are varied and include terms such as autoimmune sialadenitis, sicca complex, Mikulicz disease, and chronic sclerosing sialadenitis. One potential outcome of the lymphocytic proliferation in Sjögren syndrome is progression to lymphoma.
This chapter also covers other systemic immune and inflammatory disorders—namely, chronic graft-versus-host disease, sarcoidosis, and human immunodeficiency (HIV) infection—in which salivary gland involvement may be prominent.
Sjögren Syndrome (Autoimmune Sialadenitis, Mikulicz Disease, Chronic Sclerosing Sialadenitis)
Definition
Sjögren syndrome is named after Henrik Sjögren (1899–1986), a Swedish ophthalmologist who in a classic doctoral dissertation in 19331 reported the detailed clinical and histological findings in 19 women with xerostomia and keratoconjunctivitis sicca, 13 of whom had chronic arthritis. In 1892, however, Mikulicz2 had already reported a man with bilateral parotid and lacrimal gland enlargement associated with massive round cell infiltration, leading to the term “Mikulicz disease.” In 1953, Morgan and Castleman3 established that Sjögren syndrome and Mikulicz disease are the same entity. The term “Mikulicz disease” is therefore noted here for historical reasons. It is an obsolete term for any enlargement of the parotid gland that is not due to tuberculosis, leukemia, or some other identifiable disease.
The entity known as chronic sclerosing sialadenitis (Küttner tumor) dates from 1896,4 when it was first described as a disease manifesting in hardness and swelling of the submandibular gland. Clinically, the “tumor” lesion is indistinguishable from a true neoplasm. The diagnosis is usually made after removal of the gland and histological examination. However, there are pathogenic correlates between this lesion in the submandibular gland and autoimmune sialadenitis in Sjögren syndrome. Chronic sclerosing sialadenitis therefore most probably represents the submandibular gland equivalent of Sjögren syndrome.
Epidemiology and Etiology
As with many of the systemic rheumatic diseases, the diagnosis of Sjögren syndrome cannot be readily made clinically. During the last three decades, various national and international groups have developed multiple sets of criteria for Sjögren syndrome, giving rise to differing research results and epidemiological data.
Classification by the American–European Consensus Group criteria
After a thorough process of validation and re-validation, the American–European consensus criteria (AECC) for Sjögren syndrome, published in 2002 (Table 16.1),5 has gained wide acceptance, and the criteria are also increasingly being used for guidance in the clinical diagnosis of Sjögren syndrome. In addition to sicca symptoms in the eyes or mouth and measurements of reduced exocrine function, evidence of autoimmunity is required—either based on autoantibodies to Sjögren syndrome autoantibody A (SSA) or Sjögren syndrome autoantibody B (SSB) or a positive biopsy of the minor salivary glands of the lower lip, with a focus score of one or more.
|
I |
Ocular symptoms: a positive response to at least one of three questions |
|
II |
Oral symptoms: a positive response to at least one of three questions |
|
III |
Ocular signs—that is, objective evidence of ocular involvement, defined as a positive result for at least one of the following two tests: 1. Schirmer test, performed without anesthesia (≤ 5 mm in 5 minutes) 2. Rose bengal score or other ocular dye score (≥ 4 according to van Bijsterveld’s scoring system) |
|
IV |
Histopathology: in minor salivary glands (obtained through normal-appearing mucosa) focal lymphocytic sialoadenitis, evaluated by an expert histopathologist, with a focus score ≥ 1, defined as several lymphocytic foci (which are adjacent to normal-appearing mucous acini and contain more than 50 lymphocytes) per 4 mm2 of glandular tissue. |
|
V |
Salivary gland involvement: objective evidence of salivary gland involvement, defined by a positive result for at least one of the following diagnostic tests: 1. Unstimulated whole salivary flow (≤ 1.5 mL in 15 minutes) 2. Parotid sialography showing the presence of diffuse sialectasias (punctate, cavitary or destructive pattern), without evidence of obstruction in the major ducts 3. Salivary scintigraphy showing delayed uptake, reduced concentration, and/or delayed excretion of tracer |
|
VI |
Autoantibodies: presence in the serum of the autoantibodies to Ro (SSA) or La (SSB) antigens, or both |
|
Revised rules for classification |
|
|
Primary SS |
In patients without any potentially associated disease, primary SS may be defined as follows: a) The presence of any four of the six items is indicative of primary SS, as long as either item IV (histopathology) or VI (serology) is positive b) The presence of any three of the four objective criteria items (that is, items III, IV, V, VI) c) The classification tree procedurea represents a valid alternative method for classification, although it should be more properly used in clinical-epidemiological surveys |
|
Secondary SS |
In patients with a potentially associated disease (for instance, another well-defined connective-tissue disease), the presence of item I or item II plus any two from among items III, IV, and V may be considered as indicative of secondary SS |
|
Exclusion criteria |
Past head and neck radiotherapy Hepatitis C infection Acquired immunodeficiency disease (AIDS) Preexisting lymphoma Sarcoidosis Graft-versus-host disease Use of anticholinergic drugs (for a time less than four times the half-life of the drug) |
a Not shown in this table.
 Sjögren syndrome autoantibody A (SSA) is also known as anti-Ro, and Sjögren syndrome autoantibody B (SSB) is also known as anti-La, after the first patients who presented with these autoantibodies (Robert and Lane, respectively).
Sjögren syndrome autoantibody A (SSA) is also known as anti-Ro, and Sjögren syndrome autoantibody B (SSB) is also known as anti-La, after the first patients who presented with these autoantibodies (Robert and Lane, respectively).
Sjögren syndrome occurs throughout the world. Regional differences have not been studied. There is a large female preponderance, with a female–male ratio of about 9: 1. The disease is found in all age groups, but usually commences between the ages of 40 and 60 and is rarely seen in children and adolescents. There is often an interval of several years from the start of symptoms to confirmation of the diagnosis.
As a consequence of the different criteria used to diagnose Sjögren syndrome, prevalence estimates have varied widely, with some studies reporting up to 3% of the population being affected. Recent studies based on the American–European consensus criteria (AECC) show prevalences of approximately 0.1%, with confidence intervals in the range of < 0.1% to 0.4%.6,7 There have been few studies on the incidence of Sjögren syndrome, but according to the latest criteria from 2002, the incidence rate is more likely to be in the range of 3–6 per 100 000 population per year.
First-degree relatives with an autoimmune disease, previous pregnancies, and female gender have been identified as epidemiological risk factors for the development of Sjögren syndrome.8
 Sjögren syndrome is most common in women aged 40–60 and has a prevalence of approximately 1–4 per 1000 population.
Sjögren syndrome is most common in women aged 40–60 and has a prevalence of approximately 1–4 per 1000 population.
Secondary Sjögren Syndrome
Secondary Sjögren syndrome is the occurrence of signs and symptoms of Sjögren syndrome in conjunction with other autoimmune systemic diseases such as rheumatoid arthritis and systemic lupus erythematosus. The most common primary disease is rheumatoid arthritis, with a prevalence of secondary Sjögren syndrome of 10%–30%.
Lymphoepithelial Lesions and Lymphoid Neogenesis
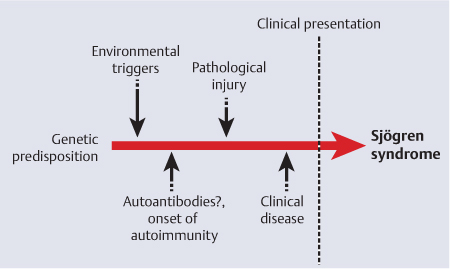
Among the possible etiological and triggering factors involved in the etiology of Sjögren syndrome, the possibility of viral infections causing the autoimmune reactions has been considered for decades. Viral triggers might include several different viruses, including Epstein–Barr virus, which has been widely studied in Sjögren syndrome.9 However, progress in this field has been slow, and no clear-cut evidence has been produced. A genetic predisposition to Sjögren syndrome has also been suggested,10 but a combination with a link to an environmental trigger remains to be defined (Fig. 16.1).
Fig. 16.1 Proposed etiopathogenic events preceding the clinical presentation and diagnosis of Sjögren syndrome.
The pathognomic histological finding in glandular biopsies is of progressive focal infiltration of mononuclear lymphoid cells, replacing the glandular epithelium (lymphoepithelial lesion). This correlates broadly with symptoms of reduced salivary secretion. However, the mechanisms leading to the salivary gland tissue being the target, the resultant accumulation of infiltrating cells, and the biological role of the cellular infiltrate are still undefined. The infiltrating cells interfere with glandular function at several levels: destruction of glandular structures by cell-mediated mechanisms; secretion of cytokines that activate pathways related to interferons (IFNs); local production of autoantibodies, etc.
The focal infiltration consists mainly of T cells, but also includes macrophages and plasma cells. Ordinarily, lymphocytes circulate in the blood and invade tissues as a response to infection or injury. This is a complex process regulated by a range of adhesion molecules on the inflammatory cell surface and the tissue endothelial cells. The lymphocytes adhere to the endothelium by means of the adhesion molecules and can move from the circulation to the tissue. Interestingly, serum levels of the adhesion molecule related to the epithelial cells, E–cadherin, have been found to be raised in Sjögren syndrome, indicating the close interaction between epithelial cells and lymphocytic organization.
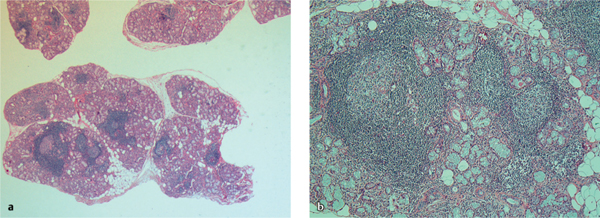
Recent studies have identified germinal center–like structures (Fig. 16.2), which have been identified in up to one-third of salivary gland samples analyzed11 and coincide with elevated titers of rheumatoid factor, other autoantibodies, increased immunoglobulin G (IgG) levels, and a higher focus score (see Table 16.1, IV). These results nevertheless suggest that the formation of ectopic lymphoid microstructures in nonlymphoid organs is involved in the pathogenesis of Sjögren syndrome. Involvement of the salivary glands, as a site of ectopic germinal center formation, and the selection of high-affinity autoantibodies12 mediating this autoimmune condition suggest novel targets for future immunomodulatory treatment strategies.
Cytokines and Chemokines
Patients with primary Sjögren syndrome have an activated type I interferon system.13 Although viruses may initiate the production of IFN, the continued IFN-α synthesis is caused by RNA–containing immune complexes that activate plasmacytoid dendritic cells to prolong IFN-α production at the tissue level.13 The finding that U1snRNA and hY1RNA are capable of inducing IFN-α indicates that immune complexes containing such RNA may be at least partly responsible for the ongoing IFN-α production seen in Sjögren syndrome.14 Activation of IFN pathways and the presence of plasmacytoid dendritic cells were also verified in a subsequent study,15 supporting the notion that interaction between the innate and adaptive immune systems is central in the pathogenesis of Sjögren syndrome.
Accordingly, the infiltration of lymphocytes into glandular aggregates plays a central role in the tissue pathology of Sjögren syndrome. The process appears to be tightly regulated, at least in part by chemokines and the local expression of their receptors. Studies of chemokine patterns have pointed further to the role of epithelial cells in the pathogenesis of Sjögren syndrome.16 This offers new insights into the mechanisms of leukocyte attraction and formation of secondary lymphoid tissue structures. In particular, the B cell–attracting chemokine CXCL13, required for normal polarization of germinal centers, has been implicated as a key regulator of lymphoid neogenesis. However, the chemokines CXCL13, CCL21, and CXCL12 do not display any typical pattern for germinal center formation in situ.17
Fig. 16.2 a, b Microscopic views of chronic inflammation in the minor salivary glands, with tissue from the inside of the lower lip of a patient with Sjögren syndrome.
a Multiple focal infiltrates.
b A high-powered view of organized lymphoid tissue (ectopic germinal centers).
B–cell activating factor (BAFF) is a member of the tumor necrosis factor (TNF) superfamily and is a crucial factor in B–cell survival and differentiation. In Sjögren syndrome, disturbances in the B–cell biology and humoral immunity, including BAFF–mediated processes, have been described.18 Locally in the glands, a reduced level of apoptosis among BAFF–expressing cells may lead to longer-persisting BAFF production and thereby maintain signaling for tissue-infiltrating B cells to proliferate and supposedly to become autoantibody-producing plasma cells. In general, activity in the BAFF system together with the IFN pathway activity could be powerful systems escalating the progression and perpetuation of autoimmunity in Sjögren syndrome.
Another molecule of the same family, a proliferation-inducing ligand (APRIL), has been found to be increased in serum and is related to serological deviations and lymphoid organization in the salivary glands of patients with Sjögren syndrome.19
 Important molecules in the pathogenesis and lymphoid neogenesis of Sjögren syndrome are IFN-α, CXCL13, BAFF, APRIL, interleukin-4 (IL-4), IL-17, IL-1β, and IL-23.
Important molecules in the pathogenesis and lymphoid neogenesis of Sjögren syndrome are IFN-α, CXCL13, BAFF, APRIL, interleukin-4 (IL-4), IL-17, IL-1β, and IL-23.
Recent multiplex cytokine screenings of patients with Sjögren syndrome have in addition identified interesting cytokine profiles that correlate with organization of the lymphoid aggregates. Germinal center–positive patients had higher levels of IL-4, IL-17, IL-1β, and the IL-23 subunit IL-12p40.20 Exploration with discriminant function analysis showed that the biomarkers that had the strongest discriminatory power were CCL11 (eotaxin), IFN-γ, and BAFF.21
B Cells and Autoantibodies
Despite the dominance of T cells in the glandular lesions, Sjögren syndrome is considered to be a humoral (B cell)–driven autoimmune disease.22 The cells of the immune system work in concert with other immunocompetent cells, and the surrounding epithelial cells may be involved in the disease process, but still the B cells have a prominent role in this disease. This view is based on serum hypergammaglobulinemia, autoantibodies, rheumatoid factor, focal B–cell infiltrations, and in some cases the development of B–cell lymphoma (non-Hodgkin lymphoma).
A characteristic feature in both primary and secondary Sjögren syndrome patients is the presence of circulating autoantibodies against the non–organ-specific Ro ribonucleoprotein (RNP) complex. The Ro-RNP complexes consist of both cytoplasmic RNA (hYn), Ro and La, and other polypeptides.23,24 The cellular function of these Ro-RNP complexes is most likely to be involved in post-translational modification and regulation. Consequently, anti-Ro and anti-La autoantibodies have become important markers in the classification criteria for SS.5
Whether these antibodies have a direct pathological effect is not known, but they have been found to be associated with specific symptoms such as sicca complaints, hypergammaglobulinemia, lymphopenia, congenital heart block, and severe salivary gland disease. There is some evidence strongly supporting a direct pathological role of anti-Ro autoantibodies. In neonatal lupus, maternal anti-Ro IgG crosses the placenta and is deposited in the fetal myocardium and skin, causing congenital heart block and skin lesions as the major symptoms.25,26 There is no direct connection between these autoantibodies and Sjögren syndrome, but plasma cells producing anti-Ro52, anti-Ro60, and anti-La48 have been identified in the salivary glands,27 and an increased concentration of autoantibodies is detected in SS patients.28
 Anti-Ro and/or anti-La are associated with sicca complaints, hypergammaglobulinemia, lymphopenia, congenital heart block, and severe salivary gland disease.
Anti-Ro and/or anti-La are associated with sicca complaints, hypergammaglobulinemia, lymphopenia, congenital heart block, and severe salivary gland disease.
Clinical Features
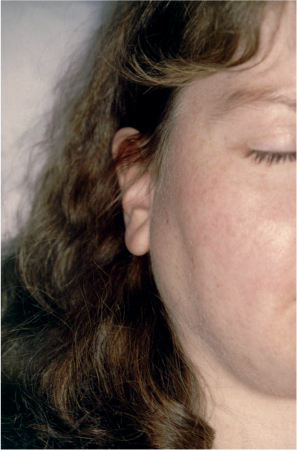
The hallmarks of Sjögren syndrome are the sicca symptoms from the eyes and mouth. Dryness of the eyes may be experienced as a gritty sensation, soreness, or intolerance of contact lenses. Dryness of the mouth may give rise to difficulties in swallowing dry foods without fluid and a need for frequent small sips of water, not only during the day but also at night. Loss of the protective and antimicrobial properties of saliva may increase dental caries and predispose for oral candidiasis. In addition, patients may have other symptoms related to dryness of the mucosal membranes or of the skin—for instance, nasal crusts and epistaxis (a bloody nasal discharge), hoarseness and dysphagia, halitosis, a reduced sense of smell and taste, dry cough, intermittent parotid swelling (Fig. 16.3) or submandibular swelling, and dyspareunia. Dry skin and dry hair are also common symptoms in Sjögren syndrome patients.
Sjögren syndrome must also be regarded as a systemic disease, as well as being dominated by local symptoms. Many patients have problems with fatigue and with joint and muscular pain, as well as nonspecific neurological complaints. Extraglandular conditions are less common, but include arthritis, which is usually nonerosive, Raynaud phenomenon, skin vasculitis, lymphadenopathy, serositis, pulmonary fibrosis, symptoms from the central nervous system, and renal tubular acidosis. Comorbidity in the form of thyroid diseases may be diagnosed in up to one-third of the patients.29–32
Fig. 16.3 Parotid gland enlargement in Sjögren syndrome.
 The clinical hallmarks of Sjögren syndrome are xerostomia and/or keratoconjunctivitis sicca, dental caries (often atypical), oral candidiasis, and fatigue.
The clinical hallmarks of Sjögren syndrome are xerostomia and/or keratoconjunctivitis sicca, dental caries (often atypical), oral candidiasis, and fatigue.
Diagnostic Work-Up
Reduced exocrine function or sicca symptoms may be caused by a variety of conditions and may also be age-related. Evidence of autoimmunity, as outlined in the most recent AECC criteria,5 help distinguish Sjögren syndrome from these other conditions. The AECC is increasingly being used for guidance in the clinical diagnosis of Sjögren syndrome. In the AECC, four of six criteria are needed to establish a diagnosis. At least one of the four must be autoantibodies or a positive biopsy (Table 16.1).
Reduced tear flow can be assessed with the Schirmer test, a measurement of wetting during 5 minutes on a 30-mm filter strip placed over the brim of the lower eyelid. Less than 5 mm of wetting is considered abnormal. Sicca symptoms in the eye may also be caused by changes in the physicochemical properties or quality of the tears, for which the tear film break-up time test is indicative. The number of corneal abrasions is another measurement for dry eyes.
Dryness of the mouth may be related to saliva production, which is measured by the unstimulated salivary flow rate. The patient is asked to collect all of the saliva produced during 15 minutes into a vial. A volume of less than 1.5 mL is considered abnormal.
Improvements in technology have taken place in recent years and new imaging techniques have been developed. Other traditional methods such as scintigraphy, as well as computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) are also needed. Such approaches to evaluate inflammation can be effective, but only if the strengths and limitations of each are taken into account and if they are carried out by experienced personnel. Imaging techniques in particular have undergone rapid development in recent years. Ultrasound has a prime position in many clinics and is directly handled by the clinician in charge. CT and MRI have also acquired important roles. In patients with xerostomia, quantitative MRI can distinguish between those with or without Sjögren syndrome.33 Sialoendoscopy may also now be acquiring an important role in both the diagnosis and treatment of salivary gland diseases.34
The most definitive test is a biopsy of the minor salivary glands from the inside of the lower lip (Fig. 16.4). After local anesthesia, an incision 1.5–2.0 cm long is made parallel to the vermilion border in the middle of the lower lip, between the midline and the corner of the mouth. At least five lobes of labial glands are then obtained by blunt dissection. After routine histological fixation and preparation, the biopsy is evaluated according to a method in which a focus is defined as an accumulation of at least 50 inflammatory cells per 4 mm2 (Table 16.1). According to the AECC, a biopsy is positive if the focus score is more than or equal to one per 4 mm2. Occasionally, islands of degenerating epithelium (lymphoepithelial lesions) together with chronic inflammatory cells are seen in labial gland biopsies, but such structures are more common in the major glands. Parotid biopsy is used in some institutions.35
Autoantibodies to the SSA or SSB antigens are also strongly indicative of Sjögren syndrome, although they can also be found in other systemic diseases such as systemic lupus erythematosus. More than 50% of Sjögren syndrome patients have one or more of these autoantibodies. Other autoantibodies such as antifodrin and anti-M3 receptor antibody have not yet met with international acceptance. Laboratory testing may also show that some patients have an increased erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia and rheumatoid factors may also be found.29
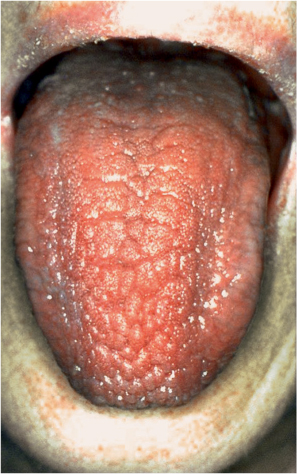
The clinical examination may reveal a dry and lobulated tongue (Fig. 16.5), fissures in the corners of the mouth, dry skin, and in some cases parotid swelling (see Fig. 16.3) or submandibular swelling, lymphadenopathy, palpable purpura (especially in the lower extremities), and arthritis.
Treatment
Palliative therapy includes the application of topical products such as tear and saliva substitutes, which help reduce the dryness and protect the cornea (from damage), as well as protecting the oral mucous membranes (from fungal infection) and teeth (from dental caries). Pilocarpine hydrochloride helps stimulate residual salivary secretion in patients in whom not all of the glandular tissue has been destroyed. Administration of local estrogens may be beneficial for genital dryness, and skin ointments for desiccated skin (Table 16.2).
Fig. 16.4 A labial salivary gland biopsy after an initial incision through the oral mucosa and blunt dissection, revealing the individual minor salivary glands. It should be noted that nerves and blood vessels traversing the area should be avoided.
Fig. 16.5 Oral dryness, with a severely dry tongue and lips in a patient with Sjögren syndrome.
|
Saliva stimulation |
|
Local
|
|
Systemic
|
|
Acupuncture |
|
Saliva substitutes |
|
|
|
Use of bedside humidifier during sleeping hours |
Systemic treatment in Sjögren syndrome is preferably handled by a rheumatologist. Nonsteroidal anti-inflammatory drugs and antimalarials are the treatments of choice for arthralgia or mild arthritis. The fatigue and myalgia in Sjögren syndrome do not usually respond to pharmaceutical measures. More severe Sjögren syndrome with high inflammatory activity, systemic or internal organ involvement, or erosive arthritis can be treated with immunomodulatory drugs and corticosteroids, in line with systemic lupus erythematosus or rheumatoid arthritis. Various biological treatments have been explored in small groups of patients. The results of anti-TNF-α treatment have been disappointing, while results with B–cell depletion (anti-CD20 treatment, rituximab) require additional controlled studies.36 All of these systemic treatments have no effect, or very limited effect, on salivary secretions.
 Surgery can be used to remove minor salivary submucosal glands from the lower lip, but direct biopsies sometimes also have to be taken from the parotid gland, and superficial or even total parotidectomy is sometimes needed for chronic parotid sialadenitis.
Surgery can be used to remove minor salivary submucosal glands from the lower lip, but direct biopsies sometimes also have to be taken from the parotid gland, and superficial or even total parotidectomy is sometimes needed for chronic parotid sialadenitis.
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 To diagnose dry eyes, the Schirmer test, tear film break-up time, and/or observations of corneal abrasions are useful. To diagnose dry mouth, the salivary flow rate, imaging findings (ultrasound, computed tomography, magnetic resonance, sialography, scintigraphy, and sialoendoscopy), and/or a labial gland biopsy are of major help.
To diagnose dry eyes, the Schirmer test, tear film break-up time, and/or observations of corneal abrasions are useful. To diagnose dry mouth, the salivary flow rate, imaging findings (ultrasound, computed tomography, magnetic resonance, sialography, scintigraphy, and sialoendoscopy), and/or a labial gland biopsy are of major help.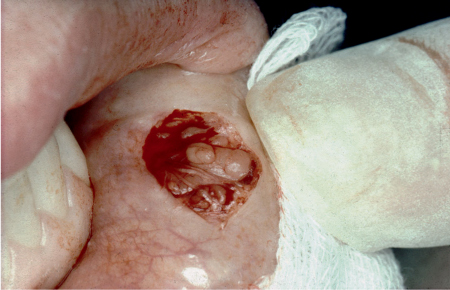
 Gustatory/masticatory stimulation with sugar-free/fluoride-containing tablets, xylitol-containing mints/lozenges, chewing gum
Gustatory/masticatory stimulation with sugar-free/fluoride-containing tablets, xylitol-containing mints/lozenges, chewing gum Pilocarpine 5 mg × 4 times daily
Pilocarpine 5 mg × 4 times daily Cevimeline 30 mg × 3 times daily
Cevimeline 30 mg × 3 times daily Artificial saliva, rinses, gels, sprays
Artificial saliva, rinses, gels, sprays