Introduction
The statin class of drugs enhances osteogenesis and suppresses bone resorption, which could be a plausible biologic mechanism for mitigation of orthodontic relapse. We aimed to determine whether atorvastatin (ATV) might affect orthodontic relapse and osteoclastogenesis by modulating expression of RANKL and osteoprotegerin (OPG), crucial molecules involved in bone turnover. Furthermore, we analyzed the adverse effects of ATV on femur turnover and endochondral ossification.
Methods
Wistar rats were subjected to orthodontic tooth movement for 21 days, followed by removal of the appliance and ATV or saline solution administration. Up to 7, 14, and 21 days of ATV administration, tooth relapse was measured, and maxilla and femur sections were obtained and prepared for hematoxylin and eosin, TRAP, and immunohistochemical (RANKL and OPG) staining.
Results
ATV decreased tooth relapse ( P = 0.03) and osteoclast counts ( P = 0.04), which were positively correlated ( P = 0.006). Statin administration increased periodontal expression of OPG ( P = 0.008), but not of RANKL protein. ATV administration also enhanced growth plate cartilage thickness.
Conclusions
Statin-induced OPG overexpression reduces relapse after orthodontic tooth movement, in a phenomenon correlated with decreased osteoclast counts. This phenomenon sheds light on OPG as a molecular target that modulates maxillary bone metabolism and orthodontic relapse.
Highlights
- •
Pharmacologic bone modulation is a feasible strategy to reduce orthodontic relapse.
- •
Atorvastatin stimulates periodontal OPG expression and inhibits osteoclastogenesis.
- •
There is a positive correlation between osteoclast numbers and orthodontic relapse.
- •
Atorvastatin affects endochondral ossification of the long bones.
Despite the clinical relevance of orthodontic relapse, the cellular and molecular mechanisms involved in this event are not fully understood. Yoshida et al proposed that remodeling of the periodontal ligament fibers and alveolar bone are the main causes of relapse. In addition, Franzen et al found that orthodontic relapse and orthodontic tooth movement (OTM) are associated with similar cellular adaptations, such as increased osteoclast differentiation in compression areas. Given this background, one could argue that endogenous or pharmacologic bone modulation to inhibit osteoclast resorption and promote osteoblast neoformation may have clinically relevant effects on the regulation of OTM and relapse.
Statins are inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase, the rate-limiting enzyme within the mevalonate pathway of cholesterol biosynthesis. In addition to their cholesterol-lowering properties, statins have a series of pleiotropic and anti-inflammatory effects. Studies have suggested that statins can influence bone turnover, enhancing osteogenesis and suppressing bone resorption. These effects involve modulation of the receptor activator of nuclear kappa B (RANK), receptor activator of nuclear kappa B ligand (RANKL), and osteoprotegerin (OPG), ultimately promoting suppression of osteoclastogenesis. In the bone system, RANKL is expressed on the osteoblasts, and as it binds to the RANK receptor expressed on hematopoietic osteoclast precursors, it induces rapid differentiation of these cells to mature osteoclasts. OPG is a decoy receptor produced by fibroblasts, osteoblasts, and even osteoclasts. This molecule will compete with RANK for RANKL binding, thus inhibiting differentiation of osteoclasts and inducing their apoptosis.
Apparently, the effects of statins on orthodontic relapse have been less explored. Han et al observed that the ability of simvastatin to minimize tooth displacement was associated with decreased RANKL and increased OPG expression. They suggested an effective drug stimulation of bone neoformation, thus accelerating tooth stability and assisting the retention phase. Furthermore, Jin et al found that simvastatin increases bone volume in rats affected by periodontal disease, with decreased RANKL expression apparently involved. Although statins seems to be well tolerated in adult and young patients, long-term undesirable effects must be considered in clinical practice. For instance, in-vitro and in-vivo preclinical studies have suggested that statins increase chondrocyte proliferation and longitudinal bone growth, which may preclude their use as an orthodontic pharmacologic strategy in children.
In this study, we hypothesized that short-term atorvastatin (ATV) treatment in rats might reduce orthodontic relapse and osteoclastogenesis through modulation of RANKL and OPG expression. We also analyzed the adverse effects of ATV on long-bone turnover and endochondral ossification.
Material and methods
Thirty-six male Wistar rats, age 6 weeks, weighing approximately 330 to 340 g, were used in the experiments. The animals were housed 4 to a cage, under a 12-hour light and dark cycle, at a constant temperature of 23°C, and received food and water ad libitum. All animal handling and care procedures were conducted in keeping with internationally accepted guidelines (Guide for the Care and Use of Laboratory Animals) and were approved by the ethics committee of the School of Dentistry of Federal University of Rio Grande do Sul in Brazil (CEUA 23145).
After induction of anesthesia with ketamine (80 mg/kg) and xilazyne (5 mg/kg), a superelastic closed nickel-titanium coil spring exerting a force of 50 cN was inserted unilaterally, between the maxillary right first molar and incisors, as described in the split-mouth design. Our protocol was based on previous demonstrations that 50cN provides substantial tooth movement. The device was kept in place for 21 days to generate mesial displacement of the first molar. Whereas the maxillary right molar was used for experimental tooth movement, the maxillary left molar served as the internal control with no orthodontic tooth movement. Throughout the study, the animals were evaluated weekly for weight gain or loss, appliance breakage, and signals of gingival or other soft tissue inflammation. After 20 days of OTM, the animals were randomly divided into 2 groups: control (saline solution [SAL], n = 18) and experiment (ATV, n = 18). ATV, 15 mg per kilogram, was given daily (Medley Farmacêutica, Campinas, São Paulo, Brazil), via gavage, for 7, 14, or 21 days ( Fig 1 , A ). Rats in the control group received 0.1 mL of phosphate-buffered saline, daily, via gavage. On day 21, the appliance was removed, marking the start of the relapse phase ( Fig 1 , A ). The experimental time points were set at 7, 14, and 21 days after appliance removal (Re7, Re14, and Re21, respectively).
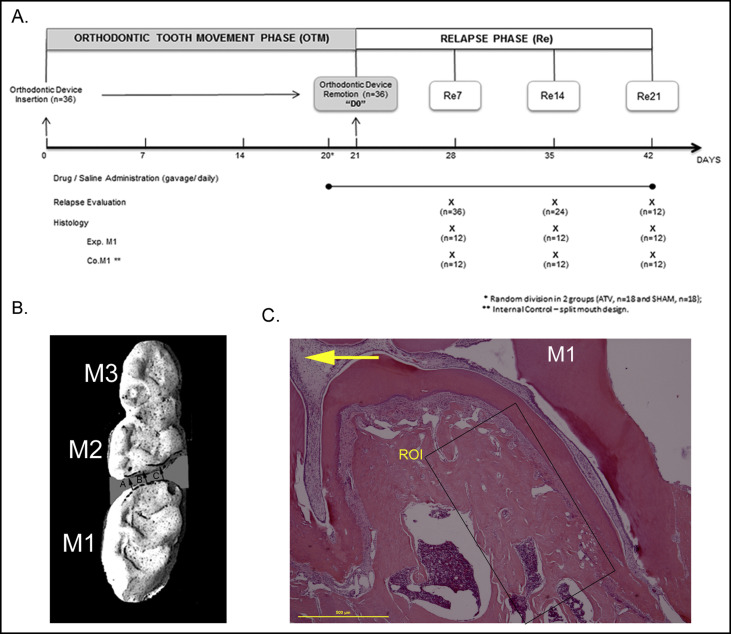
Using dental stone (Durone; Dentsply, York, Pa), precise plaster models of the maxilla were obtained from impressions made with silicone material (Perfil; Vigodent, Rio de Janeiro, São Paulo, Brazil). Impressions were obtained every 7 days under anesthesia ( Fig 1 , A ) in both groups. The occlusal surfaces were photographed (DSC#H10; Sony, Tokyo, Japan) at 300 dpi and magnified (4 times) using Image J software (version 1.44; National Institutes of Health, Bethesda, Md; 2011). A 100-mm ruler was placed next to the casts to calibrate measurements. The mean distance between the distal surface of the first molar and the mesial surface of the second molar, measured at 3 distinct points on each photo, was considered for analyses ( Fig 1 , B ). Total tooth movement during the 21 days of orthodontic treatment was recorded (baseline). At 7, 14, and 21 days after appliance removal, the distances between the first and second molars were measured and recorded. Based on the baseline and the final measurements, the percentage of relapse in each animal was calculated, and the values were averaged for each group, as described in previous studies.
At each experimental time point, 12 animals (6 per group) were killed with an overdose of ketamine and xylazine. The maxillae and distal left femurs were immediately dissected and fixed by submerging for 24 hours in 10% buffered formalin. The specimens were demineralized in 10% EDTA (pH 7) for 30 to 60 days. The samples were then dehydrated through an ethanol series and embedded in paraffin. Fifteen semisuccessive cross-sections were cut at 5 μm, and the sections numbered 1, 5, 10, and 15 were selected for staining with hematoxylin and eosin, tartrate-resistant acid phosphatase (TRAP), and immunohistochemicals (RANKL and OPG). The cut plane was considered satisfactory when the overall length of the maxillary mesial root was observed (cervical to apical region). Furthermore, the distal root of the first molar also was observed.
Briefly, for TRAP staining, histologic sections were selected and incubated in acetate buffer (pH 5.0) containing naphthol AS-MX phosphate (Sigma-Aldrich, St Louis, Mo), Fast Red Violet LB Salt (Sigma-Aldrich), and 50 mmol per liter of sodium tartrate. The sections were counterstained with hematoxylin.
Histomorphometric analyses of the maxilla specimens were performed considering the following subgroups: experimental hemimaxillae from the ATV animals, control hemimaxillae from the ATV animals, experimental hemimaxillae from the SAL animals, and control hemimaxillae from the SAL animals. Comparisons across subgroups were performed at each time point (7, 14, and 21 days) or by analysis of overall means. For evaluation of the femur specimens, the sample was divided into 2 groups: ATV (n = 18) and SAL (n = 18). In this case, all comparisons between groups were done considering overall mean values.
Hematoxylin and eosin and TRAP stained sections were visualized in an Eclipse microscope (90i; Nikon, Tokyo, Japan) coupled to a Coolsnap EZ camera (Photometrics, Tucson, Ariz). Microphotographs were captured using the NIS Elements Imaging 3.10 Sp2 software (Nikon). All histomorphometric measurements and terminology were in accordance with the American Society for Bone and Mineral Research recommendations.
Molar-supporting structures were evaluated using the NIS Elements Imaging 3.10 Sp2 software. Under high magnification (100 times), the number of osteoclasts was counted. The region of interest consisted of the periodontal tissues at the distal surface of the mesial root of the first molar ( Fig 1 , C ). The mesial root of the maxillary first molar was selected because it is the largest of the 5 roots in this rat’s teeth, thus allowing histologic evaluation of the entire root structure. Furthermore, it is commonly used for analysis in tooth-movement studies. According to Gonzalez et al, during rats’ OTM, a major stress occurred in the periodontal tissues adjacent on the middle part of the mesial root. We tend to argue that the region of interest could represent the pressure zone during the relapse phase. Cells were considered to be osteoclasts if they were TRAP-positive, multinucleated, and located on the bone surface or residing in Howship’s lacunae.
The maxillary bone volume ratio (BV/TV), expressed as the ratio of cancellous bone volume (BV) to total tissue volume (TV), was defined in the region of interest of the hematoxylin and eosin-stained sections ( Fig 1 , C ) using Adobe Photoshop CS6 software (Adobe Systems, San Jose, Calif) and ImageJ, following the method suggested by Egan et al. The total osteoclast count and bone volume were calculated for each animal and averaged for each group.
For femur bone turnover, the number of osteoclasts was counted in 10 randomly selected fields of view in the metaphyseal regions of the femur specimens, using the same software described above. The BV/TV ratios of 5 rectangular areas of subchondral bone tissue were calculated according to the protocol outlined by Ho et al. Again, the total osteoclast count and bone volume were calculated for each animal and averaged for each group.
For endochondral ossification, growth plate cartilage and hypertrophic zone thickness were calculated as the means of 10 measurements performed at randomly selected locations in hematoxylin and eosin-stained sections of the femur, using the NIS Elements Imaging 3.10 Sp2 software.
Immunohistochemical staining was performed on each hemimaxilla section (with and without OTM) after mounting on silanized slides (Dako, Glolstrup, Denmark). After deparaffinization and dehydration, endogenous peroxidase activity was blocked using hydrogen peroxide (30 volume) and methanol (1:1) for 10 minutes. Antigen retrieval was performed using trypsin (0.25%) for 20 minutes at room temperature. Sections were then incubated overnight with primary antibodies in a humidified chamber at 4°C. The antibodies and conditions were as follows: RANKL, rabbit polyclonal antibody (Bioss, Woburn, Mass; bs-0747R, dilution 1:200); OPG, rabbit polyclonal antibody (Abcam, Cambridge, United Kingdom; ab73400, dilution 1:200). Immunodetection was performed using the EnVision + Dual Link system-HRP (K4063; Dako) and diaminobenzidine as substrate-chromogen system (K3468; Dako). Harris hematoxylin (Sigma-Aldrich) was used as the counterstain. A negative control, which consisted of primary antibody omission, was included in all reactions, as was an internal positive control (bone marrow).
Digital images representing the periodontal region of interest were obtained at magnification of 200 times. For each image, the color deconvolution method was used to isolate RANKL and OPG positive diaminobenzidine-stained cells/stroma. Diaminobenzidine and hematoxylin nuclear staining were digitally separated using ImageJ software and an ImageJ plug-in for color deconvolution, which calculated the contribution of diaminobenzidine and hematoxylin. After deconvolution, the diaminobenzidine image was transformed into an 8-bit format and the threshold set to black and white, with the black pixels considered as positive diaminobenzidine staining. The total value of black pixels observed in the region of interest was calculated for each animal and then averaged for each group, ATV (n = 18) or SAL (n = 18). The overall mean RANKL- and OPG-stained areas were compared between the SAL and ATV groups in the experimental and control hemimaxillae (with and without OTM, respectively).
All procedures were performed by a blinded and calibrated examiner (G.D.). To determine random intraindividual error, 10% of the sample (plaster models and histologic sections) was randomly selected for reevaluation 15 days after the first analysis. The Dahlberg formula was applied, and acceptable values were obtained (<10%).
Statistical analysis
Data are presented as means and standard deviations or, when appropriate, standard errors. Relapse was calculated as a percentage per group, and a linear mixed model with repeated measures for time was used. At each time point, between-subgroup comparisons of maxillary histomorphometric parameters were analyzed with 1-way analysis of variance (ANOVA) followed by the least significant difference multiple comparison test (for homogeneous variances) or the Games-Howell test (for heterogeneous variances). Pearson correlation coefficients were used to verify the association between orthodontic relapse and osteoclast count. When comparisons were performed between 2 groups, the independent Student t test was used. Results were processed with PASW Statistics for Windows (version 18.0; SPSS, Chicago, Ill). The significance level was set at 5% ( P <0.05).
Results
There was no between-group difference ( P >0.05) in weight gain or loss during the OTM period (days 0-21) or during the relapse phase (days 21-42). Mean (SD) weights at the end of the relapse phase were 311.67 g (66.15 g) for the SAL group and 349.17 g (30.44 g) for the ATV group.
The SAL animals reached maximal relapse at day 7 (31.91%), and the percent of relapse decreased gradually thereafter through days 14 to 21 (29.36% and 28.40%, respectively). The ATV animals exhibited reduced relapse at 7, 14, and 21 days (9.59%, 20.59%, and 7.94%, respectively) when compared with the SAL group, indicating that ATV administration prevents orthodontic relapse independently of the time point of assessment ( Fig 2 ).
ATV administration significantly reduced osteoclast counts and increased BV/TV ratios during the orthodontic relapse period ( Fig 2 , B and C ). However, when the different time points of the relapse period were analyzed separately (7, 14, and 21 days), we observed a transient osteoclast inhibition in association with statin administration. Only after 7 days of relapse (Re7) did ATV promote a marked decrease in the number of TRAP+ cells ( P <0.05) when compared with SAL + OTM animals and the control hemimaxillae (without OTM) of both groups (SAL and ATV) ( Fig 3 , A and B ). A positive and significant correlation between osteoclast count and orthodontic relapse was confirmed ( r = 0.452; P = 0.006; r 2 = 0.204). Control hemimaxillae (without OTM) from the ATV animals also exhibited reduced osteoclast counts when compared with the control hemimaxillae from the SAL group; however, this difference was not significant ( P >0.05).
In the absence of mechanical force (hemimaxillae without OTM), the BV/TV ratio was higher in the ATV than in the SAL animals ( Fig 3 , C and D ), indicating an effect of ATV administration on bone turnover under physiologic conditions. When analyzing the BV/TV ratio at each time point of the relapse phase (7, 14, and 21 days), a statistical increase in bone volume in the ATV specimens when compared with the control hemimaxillae of the SAL animals was observed only after 14 days ( Fig 3 , C and D ).
At the molecular level, ATV affected OPG expression in periodontal tissues. Overexpression of OPG was observed in both ATV subgroups when compared with their corresponding SAL controls, as shown in Figure 4 , A and B . Our observational data suggest that fibroblasts, osteoblasts, bone lining cells, and even osteoclasts had cytoplasmic OPG labeling ( Fig 4 , A ). Interestingly, cytoplasmic expression of OPG in osteoclasts was observed especially in the control hemimaxillae (without OTM) of the ATV animals. During orthodontic relapse, these cells appeared to lose the cytoplasmic OPG labeling capability ( Fig 4 , A ). Furthermore, both during orthodontic relapse and under physiologic conditions, RANKL expression was decreased in the ATV groups when compared with the SAL animals ( Fig 4 , C ), although the difference was not statistically significant ( Fig 4 , D ).




