CAS no.
80-05-7
Organic compound
Bisphenol A (2,2-bis-(4hydroxyphenyl)propane)
Chemical structure
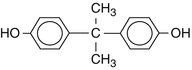
Formula
C15H16O2
Molecular weight
228.29 g/mol
Boiling point
398 °C at 760 mmHg
Melting point
153–157 °C
pKa
9.6–11.3
Water solubility
120–300 mg/L
Vapor pressure
0.2 mmHg at 170 °C
Log Kow
2.20–3.82
Henry’s constant
1.0 × 10−10 atm m3/mol
Bisphenol A is a chemical manufactured in large quantities. It is estimated that 1,150 tonnes/year are produced and used in Western Europe. Almost 96 % of BPA is used as a monomer for the production of polycarbonate and epoxy resins. Other applications include its use as stabilizing agent in plastics, as antioxidant in tire production, and as basic chemical in the production of certain flame retardants. The BPA-based materials are used in food and beverage containers, protective coating, automotive lenses, optical lenses, adhesives, powder paints, building materials, compact disks, thermal paper, paper coatings, dental, surgical, and prosthetical materials [3, 4]. The production and extensive use of these materials result in the release of this compound into the environment during processing, handling, and transportation of final products. It was estimated that 39.5 % of the total environmental release of BPA comprised total air release, 1 % water release, 54 % land release, and 5.4 % underground injection [3].
Various in vitro and in vivo assays showed that BPA presents estrogenic activity, and consequently, it is considered as important organic pollutant [3]. BPA may cause a variety of adverse effects on reproduction and development of exposed organisms, being more striking and irreversible during embryonic development. These effects may occur even at doses of BPA well below those showing adverse effects in routine toxicity studies [3–7].
The US-EPA, under the Toxic Substances Control Act, indents to consider initiating rulemaking to identify BPA on the Concern List as a substance that may present an unreasonable risk to the environment on the basis of its potential for long-term adverse effects on growth, reproduction, and development in aquatic species at concentrations similar to those found in the environment [4]. BPA is candidate to be among the first substances to go through Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) in EU registration (EU Regulation No 1907/2006). Canada was the first country that has classified BPA as toxic substance and announced restriction of imports, sales, and advertising of polycarbonate baby bottles containing BPA. Recently, the European Commission published a new directive (2011/8/EU) to restrict bisphenol A in feeding bottles that are intended for use by infants under the age of 12 months [8]. According to this directive, member states are required to prohibit the manufacture of polycarbonate feeding bottles containing BPA as well as their import and sale in EU.
The study of the occurrence of BPA in various environmental compartments, in food, in dental materials, and in biological fluids contributes to the knowledge on the environmental fate of this compound, the possible pathways of exposure, the biotransformation mechanisms, and the possible risks. In order to identify and determine trace levels of BPA in complex matrices, sensitive analytical methods are required.
A number of analytical methods have been developed for the determination of BPA. The general scheme of analysis usually comprises isolation from samples through extraction, cleanup steps, and determination by employing a sensitive analytical technique. The major problems associated with the analysis are possible loss or contamination during sampling and storage, the need of preconcentration and, possibly, of cleanup, as well as the need for highly efficient separation procedures and selective detection techniques. Reliable analytical procedures require detailed method validation and careful evaluation. In addition, sampling and sample preparation should be considered integrally with the characterization of an analytical procedure.
2.2 Sampling and Storage
The first step in the measurement of BPA involves representative sampling and maintaining sample integrity prior to analysis. The sampling strategy should reflect the known or expected variability of the system.
All the equipment that may come into contact with sample or the extract should be free from interfering compounds. The sampling containers should be made of materials that do not change the sample during the contact time. Plastics and other organic materials should be avoided during sampling, sample storage, or extraction. Glass brown bottles with glass stoppers or with PTFE-lined screw caps, carefully precleaned, are recommended for sampling and storage of samples. Rinsing with acetone is recommended for all glassware used in the analysis. Alternatively, non-volumetric glassware may be heated to at least 200 °C for a minimum of 2 h. The samples should be analyzed as soon as possible; otherwise, they can be stored at 2–5 °C for 2 weeks [9].
2.3 Extraction Techniques
Sample preparation is an important stage in the analytical process when trace analyte determination is needed. The analysis of pollutants at low concentrations in complex matrices requires the elimination of interferences and the reduction of final extract volumes to attain higher preconcentration of target analytes. Generally these pretreatment methods are necessary in order to improve the detection and quantification limits, avoid matrix implications, limit background noise, and extend the life of the analytical column.
The analysis of BPA in environmental, food, and biological liquid samples employs a wide range of sample extraction techniques. Solid-phase extraction is frequently used for isolation and preconcentration of BPA (Tables 2.2, 2.3, 2.4, and 2.5). Moreover, other techniques such as the traditional liquid–liquid extraction, solid-phase microextraction, and stir bar sorptive extraction have been used.
Table 2.2
Analytical methods and concentration range of BPA in dental materials
|
Dental materials
|
Samples analyzed/pretreatment
|
Analytical method
|
LOD
|
Concentrations of BPA
|
Reference
|
|---|---|---|---|---|---|
|
Core build-up materials
|
Eluates in ethanol 75 %
|
LC-MS/MS
|
0.5 μg/mL
|
BDL-6.14 μg/mL
|
Brenn-Struckhofova and Cichna-Markl [43]
|
|
Column: CC 125/4 Nucleodur 100-5 C18
|
|||||
|
Mobile phase: 0.1 % formic acid/acetonitrile
|
|||||
|
Diagnostic ions: m/z 227
|
|||||
|
Orthodontic adhesives
|
Eluates in alcohol 99 %
|
HPLC-UV/Vis
|
0.1 ppm
|
BDL
|
Fontana et al. [57]
|
|
Column: C18
|
|||||
|
Mobile phase: acetonitrile/water (60:40 v/v)
|
|||||
|
Wavelength: 228 nm
|
|||||
|
Dental sealants
|
Eluates in ethanol 95 %
|
HPLC-UV
|
0.0001 μg/mg
|
BDL
|
Cunha et al. [58]
|
|
Column: Nova Pak C18
|
|||||
|
Mobile phase: A acetonitrile/water (50:50 v/v)
|
|||||
|
B acetonitrile
|
|||||
|
Wavelength: 215 nm
|
|||||
|
Dental sealants
|
Eluates in distilled water
|
HPLC-UV/Vis
|
–
|
BDL
|
Salafranca et al. [59]
|
|
Column: C18 resolved column
|
|||||
|
Mobile phase: isocratic 70 % methanol
|
|||||
|
Wavelength: 215 nm
|
|||||
|
Orthodontic adhesives
|
Eluates in distilled water
|
GC-MS, EI, SIM
|
2.3 ng/L
|
0.16–2.90 μg/L
|
Maragou et al. [44]
|
|
SPE (Oasis HLB)
|
|||||
|
Elution with acetone
|
|||||
|
Derivatization with BSTFA
|
|||||
|
Column: 5 % diphenyl-95 % dimethyl polysiloxane
|
|||||
|
Diagnostic ions: m/z 357.2, 358.2
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Composites/sealants
|
Vigorous agitation with distilled water (37 °C) at various pH values (1–12)
|
HPLC-UV
|
0.20 μg/mL
|
0.3–116.1 μg/mL in polymerized materials
|
[60]
|
|
Column: C18
|
|||||
|
Mobile phase: gradient A acetonitrile/water (1:1 v/v) and B acetonitrile
|
|||||
|
<0.2–179.5 μg/mL in unpolymerized materials
|
|||||
|
Wavelength: 280 nm
|
|||||
|
GC-MS
|
|||||
|
Column methyl silica
|
|||||
|
Diagnostic ions: m/z 213, 228
|
|||||
|
Dental sealants/adhesive resins
|
Eluates in water/acetonitrile (43/57)
|
HPLC-UV/Vis
|
100 pg
|
BDL
|
[60]
|
|
Reversed phase column
|
|||||
|
Mobile phase: water/acetonitrile (43/57)
|
|||||
|
Wavelength: 215 nm
|
|||||
|
GC-MS
|
|||||
|
Column: methyl silicon DB-1
|
|||||
|
Dental sealants
|
Saliva
|
GC-MD, NCI
|
0.1 ng/mL
|
0.17–96.2 ng/mL
|
Kawaguchi et al. [46]
|
|
Urine
|
Column: 5 % phenyl-methyl-polysiloxane
|
||||
|
0.6–112.2 ng/m
|
|||||
|
SPE C18
|
Diagnostic ions: m/z 407, 299
|
||||
|
Internal standard 13C12-BPA
|
|||||
|
Elution with methanol
|
|||||
|
Derivatization: pentafluoro-benzyl bromide
|
|||||
|
Composite resins
|
Saliva
|
ELISA “EIKEN”kit
|
0.3–100 ng/L
|
Shao et al. [39]
|
|
|
Dental sealants
|
Saline solution (37 °C)
|
HPLC-FLD
|
5 ppb
|
BDL
|
Chang et al. [45]
|
|
Saliva
|
Column: Supelcosil LC-C18
|
BDL
|
|||
|
Serum
|
|||||
|
SPE C18
|
|||||
|
Elution with acetonitrile
|
Mobile phase: acetonitrile/water (50:50 v/v)
|
||||
|
Exc/Emis wv: 278/315 nm
|
|||||
|
Restorative composites
|
Eluates in ethanol
|
HPLC-diode array
|
BDL-84.4 μg/100 mg 3.5–30 μg/mL
|
Kawaguchi et al. [61]
|
|
|
Dental sealants
|
Saliva
|
Column: S5 ODS
|
|||
|
Mobile phase: gradient A acetonitrile/water (1:1) and B acetonitrile
|
|||||
|
Wavelength: 235 nm
|
|||||
|
GC-MS confirmation
|
Table 2.3
Analytical methods and levels of BPA in environmental samples
|
Samples/Country
|
Pretreatment
|
Analytical method
|
LOD
|
Concentration of BPA
|
Reference
|
|---|---|---|---|---|---|
|
River water
|
LLE with dichloromethane
|
GC-MS, EI, SIM
|
0.5 pg/μL
|
17–776 ng/L
|
Heemken et al. [21]
|
|
Column: 5 % phenylmethyl silicon
|
|||||
|
BDL-249 ng/L
|
|||||
|
Sea water
|
|||||
|
Diagnostic ions: m/z 315, 331, 407
|
|||||
|
HPLC clean up
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Germany
|
Derivatization with HFBA
|
||||
|
Freshwater
|
Filtration
|
GC-MS, EI, Full-scan
|
20 ng/L
|
BDL-1, 924 ng/L
|
Quednow and Püttmann [22]
|
|
Column: BP-X5
|
|||||
|
SPE (Bod Elute OOL)
|
Diagnostic ions: m/z 213, 228
|
||||
|
Germany
|
Elution with methanol/acetonitrile
|
||||
|
Surface waters
|
SPE (LiChrolut)
|
GC-MS/MS, EI
|
0.1 ng/L
|
0.5–410 ng/L
|
Fromme et al. [23]
|
|
RP-HPLC-FLD (Exc/Emis wv 228/310 nm)
|
|||||
|
18–702 ng/L
|
|||||
|
2.0 ng/L
|
|||||
|
Sewage effluents
|
Elution with acetone
|
||||
|
Mobile phase: gradient A hexane
|
|||||
|
Germany
|
|||||
|
B hexane/methanol/isopropanol (40/45/15)
|
|||||
|
Surface waters
|
Filtration
|
GC-MS, EI, SIM
|
2.4 ng/L
|
9–76 ng/L
|
Voutsa et al. [24]
|
|
Column: DB-5
|
|||||
|
Diagnostic ions: m/z 357.2, 358.3
|
|||||
|
SPE (Oasis HLB)
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Elution with acetone
|
|||||
|
Switzerland
|
Derivatization with MSTFA/2 % Sylon BTZ
|
||||
|
Surface water
|
Filtration
|
LC-MS/MS, ESI, NI, MRM
|
1.1 ng/L
|
2–46 ng/L
|
Jonkers et al. [25]
|
|
1.3–1, 640 ng/L
|
|||||
|
Column: 100 RP18ec
|
|||||
|
Mobile phase: gradient A water 4 mM ammonium acetate B methanol
|
|||||
|
Wastewaters
|
SPE (Oasis HLB)
|
||||
|
Precursor ion: m/z 227.02
|
|||||
|
Product ion: m/z 211.8
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Switzerland
|
Elution with MTBE/2-propanol
|
||||
|
Wastewaters
|
SPE (Oasis HLB)
|
GC-MS, EI, SIM
|
0.5 ng/L
|
450 ng/L
|
Jeannot et al. [26]
|
|
Column 95 % dimethyl-5 % phenylpolysiloxane
|
|||||
|
Elution with methanol-diethyl ether (10:90 v/v)
|
|||||
|
Identification ion: m/z 358
|
|||||
|
GC-MS/MS, EI
|
|||||
|
Precursor ion: m/z 358
|
|||||
|
Product ions: m/z 191, 267, 357
|
|||||
|
France
|
|||||
|
Derivatization with BSTFA
|
|||||
|
Surface waters
|
Filtration
|
GC-MS, EI, SIM
|
2.3 ng/L
|
15–460 ng/L
|
|
|
SPE (Oasis HLB)
|
Column: 5 % diphenyl-95 % dimethyl polysiloxane
|
||||
|
Pothitou and Voutsa [29]
|
|||||
|
15–56 ng/L
|
|||||
|
Arditsoglou and Voutsa [30]
|
|||||
|
15–2, 358 ng/L
|
|||||
|
Elution with acetone
|
|||||
|
Diagnostic ions: m/z 357.2, 358.2
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Coastal waters
|
Derivatization BSTFA
|
||||
|
Wastewaters
|
|||||
|
Greece
|
|||||
|
Surface waters
|
Decantation
|
LC-MS/MS, RP, ESI, API
|
2 ng/L
|
3–175 ng/L
|
Loos et al. [31]
|
|
Column: Synergi Polar RP
|
|||||
|
SPE (Oasis HLB)
|
Mobile phase: water/acetonitrile
|
||||
|
Wastewaters
|
Elution with ethanol/acetone/ethyl-acetate (2:2:1)
|
||||
|
Precursor ion: m/z 227
|
|||||
|
Italy-Belgium
|
Product ions: m/z 133, 212
|
||||
|
Lagoon water
|
SPE (ENVI-18)
|
HPLC-MS, ESI
|
1 ng/L
|
BDL-145 ng/L
|
Pojana et al. [32]
|
|
Column: C8-2
|
|||||
|
Mobile phase: gradient A acetonitrile B water
|
|||||
|
Elution with acetonitrile, methanol, water
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Italy
|
|||||
|
Precipitation
|
LLE with dichloromethane
|
LC-MS, ESI, NI
|
5 ng/L
|
bdl-357 ng/L
|
Peters et al. [33]
|
|
The Netherlands
|
Column: Symmetry C18
|
||||
|
Surface waters
|
Filtration
|
GC-MS
|
14 ng/L
|
BDL-330 ng/L
|
Belfoid et al. [34]
|
|
Column: SGE BPX5
|
|||||
|
Diagnostic ions: m/z 357
|
|||||
|
Internal standard: BPA-d16
|
|||||
|
SPE (SDV-XC disks)
|
|||||
|
Elution with methanol
|
|||||
|
Derivatization with SIL A
|
|||||
|
The Netherlands
|
|||||
|
Wastewater
|
Filtration
|
LC-MS/MS, NI, MRM
|
2 ng/L
|
0.15–1.55 μg/L
|
Mauricio et al. [35]
|
|
5 μg/L
|
|||||
|
Column: Purospher STAR RP-18
|
|||||
|
Mobile phase: gradient A methanol B water
|
|||||
|
SPE (Oasis HLB)
|
|||||
|
Precursor ion: m/z 227
|
|||||
|
Product ions: m/z 133, 211
|
|||||
|
Internal standard: oxybenzoic acid
|
|||||
|
ELISA
|
|||||
|
Portugal
|
Elution with dichloromethane
|
||||
|
Surface waters
|
Filtration
|
HPLC-MS, ESI, NI
|
0.09 μg/L
|
BDL-2.97 μg/L
|
Céspedes et al. [36]
|
|
Column: 100RP-18
|
|||||
|
0.06–1.51 μg/L
|
|||||
|
Mobile phase: gradient A methanol B water
|
|||||
|
SPE (Lichrolut RP-18)
|
|||||
|
Diagnostic ions: m/z 227
|
|||||
|
Internal standard: 4-heptylpheno
|
|||||
|
Elution with acetonitrile
|
|||||
|
Wastewater
|
|||||
|
Spain
|
Table 2.4
Analytical methods and levels of BPA in food samples
|
Samples
|
Pretreatment
|
Analytical method
|
LOD
|
Concentration of BPA
|
Reference
|
|---|---|---|---|---|---|
|
Bottled water
|
LLE with dichloromethane
|
GC-MS, EI
|
2.3 ng/L
|
3.5–150 ng/L
|
Nathanson et al. [12]
|
|
Column: 5 % diphenyl-95 % dimethyl polysiloxane
|
|||||
|
Derivatization BSTFA
|
|||||
|
Diagnostic ions: m/z 357.2, 358.2
|
|||||
|
Internal standard BPA-d16
|
|||||
|
Bottled water
|
SPE (OASIS HLB or C18)
|
GC-MS, EI
|
0.005 μg/mL
|
bdl-0.011 μg/L
|
Inoue et al. [76]
|
|
Column: HP 5MS
|
|||||
|
Elution two steps
|
Diagnostic ions: m/z 213, 119, 228
|
||||
|
Internal standard: 4nNP
|
|||||
|
A dichloromethane/hexane (4:1 v/v)
|
|||||
|
B ethanol/dichloromethane (9:1 v/v)
|
|||||
|
Mineral water
|
SPE (OASIS HLB)
|
LC-MS/MS, ESI, NI, MRM
|
0.01 ng/L
|
BDL
|
Gallart-Ayala et al. [77]
|
|
Column: A symmetry C-18
|
|||||
|
Mobile phase: A methanol and B water
|
|||||
|
0.60 ng/L
|
|||||
|
Elution with methanol/dichloromethane
|
|||||
|
Precursor ion: m/z 227.2
|
|||||
|
Product ions: m/z 93.1, 133.4, 212.4
|
|||||
|
Soda beverages
|
|||||
|
Canned soft drink products
|
SPE (C18)
|
GC-MS, EI
|
27–74 ng/L
|
0.032–4.5 μg/L
|
Hennion [51]
|
|
Column: HP 5MS
|
|||||
|
Diagnostic ions: m/z 213, 228, 270, 312
|
|||||
|
Elution acetonitrile-water (1:1 v/v)
|
|||||
|
Internal standard: BPA-d16
|
|||||
|
Soft drinks/beers
|
DLLME
|
GC-MS, EI
|
5 ng/L
|
BDL-4.7 μg/L
|
Joskow et al. [17]
|
|
Column: HP 5HT, HP 5MS
|
|||||
|
Diagnostic ions: m/z 213, 228, 270, 312
|
|||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


