Natural estrogens
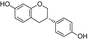
Estradiol
Estrone
Estriol
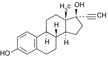
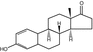
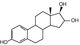
Phytoestrogens
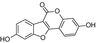
Genistein
Coumestrol
Equol (4′,7-isoflavandiol)
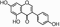
Synthetic steroid estrogens
17a-ethinylestradiol
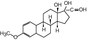
Mestranol
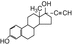
Synthetic nonsteroidestrogenic compounds
Chlorinated hydrocarbons
DDT
Methoxychlor
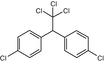
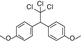
Polychlorinated Biphenyls (PCBs)
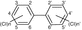
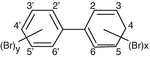
Polychlorinated Biphenyls (PBBs)
Aromatic heterocyclic compounds
PCDDs (Dioxins)
PCDFs
Polycyclic Aromatic Hydrocarbons (PAHs)
Β[a]P: Benzo-a-pyrene
DB[a,l]P: Dibenzo-[α.l]-pyrene
Aromatic Amines (AA) and Heterocyclic Aromatic Amines (HAA)
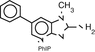
PhIP: 2amino-1-methyl-6phenylimidazolo[4,5-b]-pyridine
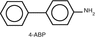
4-ABP: 4aminobiphenyl
Alkylated phenols
Octy-phenols
Nonyl-phenols
Monomers of polymeric plastics
Bisphenol-A
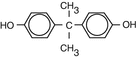
Synthetic pyrethrines
Allethrin
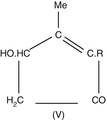
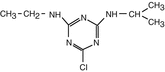
Triazines
Atrazine
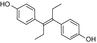
Pharmacological substances
Diethylstilbestrol (DES)
Surface water, municipal effluents from sewage treatment plants, and sediments are among the important contamination sources in many European and other countries [19–21] with consequent adverse effects in wildlife (fish, roach, etc.) [22, 23]; the major intake of estrogenic chemicals is considered to be through food [24, 25]. Fish products may represent an important dietary source of EDC contamination in food, but edible plants may also take up estrogenic compounds from terrestrial or aquatic environments [26, 27]. Note that weak estrogenicity has also been detected in mineral water and milk as a result of the leach from the polyethylene terephthalate (PTE) in baby bottles [28–30]. EDC chemicals are present in higher amounts in humans because humans are at the top of the food chain, having ingested plants and animals that contain low levels of these persisting compounds.
EDCs share physical and chemical properties such as chemical stability, lipid solubility, accumulation in fat, slow rate of biotransformation, and biodegradation. They are weak estrogens (most of them about 1/1,000 to 1/1,000,000 of the activity of estradiol), but small changes in more innocent compounds can give rise to persistent and bioaccumulative compounds (replacement of chloride by bromide leads to lipophilic brominated organic compounds that, although they show a weak estrogenic activity, tend to accumulate much more in fat compared to chlorinated ones) [31]. The major difference between naturally occurring biochemical molecules and man-made compounds is that the former are assembled and disassembled very rapidly in the human body, while the latter resist biodegradation in the environment and consequent bioaccumulation and biomagnification within various food chains. Thirteen years after Yu-cheng accident (literally oil symptoms), in which people in Taiwan had consumed PCB- and PCDF-contaminated cooking oil for 9 months (estimated consumption 1 g of PCBs and 3.8 mg of PCDFs), the concentrations in women that had born a child were 7- up to 130-fold higher (depending on the compound) compared to nonexposed population [32].
In contrast to endogenous hormones that bind to carrier proteins and thus become biologically inactive, EDCs remain unbound and active. The half-life of these compounds is ranging from weeks to years (i.e., half-life of methoxychlor is 2 weeks; of DDT, 6 months; of PCBs, PCDDs, and PCDFs, 7–10 years) [33].
Many estrogen-like compounds with high biologic activity are present in trace amounts, but since man is exposed to a plethora of these chemicals, the overall estrogenicity might be important and may contribute to overall risk and health implications [34]. Because of the long half-life and bioaccumulation of many EDCs, the “safe” concentrations today may become responsible for adverse effects in the following years [35].
1.2 Mechanism of Action of Estrogens and Xenoestrogens
1.2.1 Estrogen Receptor Signalling Pathway
The pleiotropic effects of estrogens in the body are mainly effectuated by binding to the estrogen receptors, ERα and ERβ [36], representing products of two different genes localized on human chromosomes 6 and 14, respectively [37]. Although both isotypes exist in the various systems, ERα is the main isotype in the genital system and mammary gland, while Erβ is the main isotype in the central nervous, the cardiovascular, and the immune systems; the urogenital and gastrointestinal tracts; the kidneys; and the lungs [38–42]. Various ERα and ERβ isoforms and splicing variants (hERβ1 long, hERβ1 short, hERβ2, hERβ4, hERβ5, hERα-46) have been described [43, 44].
The ERs (α, β) are composed of three independent but interacting functional domains: the NH2-terminal transcriptional AF1 (activation function-1) domain, the DNA-binding domain, and the ligand-binding domain that contains a ligand-dependent transcriptional AF2 (activation function-2) domain [45]. Although the DNA-binding domains of ERα and ERβ show a high degree of homology (only three amino acids difference), the ligand-binding domain shows only 53 % homology (Fig. 1.1).


Fig. 1.1
The functional domains of ERα (top) and ERβ (bottom) with the amino acids counting and the identity percent’s (%) are shown. The DNA-binding domain and the hinge region are highly conserved between the two receptors. AF-1 Activation function 1; DBD – DNA-binding domain; H Hinge; LBD Ligand binding domain; AF-2 Activation function 2
The classical mechanism of activation of ERs, through which genomic effects take place, depends on ligand binding to the receptors, after which the receptors dimerize and bind to estrogen response elements (EREs) located in the promoters of estrogen-responsive genes to activate gene transcription [46, 47]. ERα (but not ERβ) has also the ability to bind to the orphan nuclear hormone receptor SF-response elements (SFREs) that serve as its EREs [48].
Maximum transcriptional activity requires the concerted actions of the ligand-independent AF1 domain and the ligand-dependent AF2 domain. Regulatory cofactors of the transcriptional activity include coactivators, corepressors, and chromatin-remodeling complexes (chromatin is regulating the basal activity of many promoters) [46, 49–52] (Fig. 1.2).
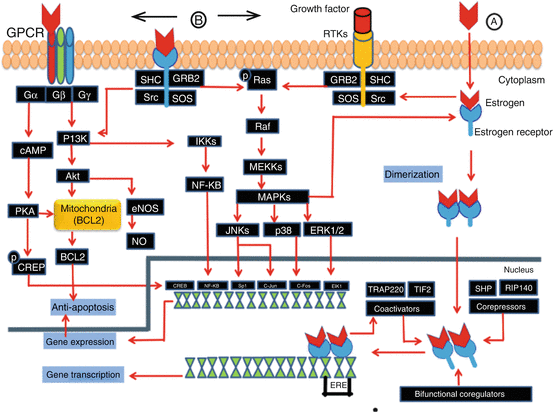

Fig. 1.2
Schematic Illustration of Classical (genomic) and Non-Classical (non-genomic) estrogen signaling pathways: (A) Classical pathway: ER complex, homodimerization and translocation from cytoplasm to to the nucleus. In the nucleus it induces two pathways: 1. Direct binding to responsive elements in the target gene promoters, subsequently the receptor-ligand complex binds to the palindromic ERE, and stimulates gene transcription with the recruitment of corregulators (coactivators, coreprossors and bifunctional coregulators). 2. ER complex interacts with transcription factors such as NF-κbB, activator protein-1 and SP1 to influence gene transcription. (B) Non-Classical pathways (non-genomic): Estrogen interaction with Nonsteroidal hormone receptors or Steroid hormone receptors in the membrane. Both non-classical pathways activate kinases that ultimately regulate transcription of specific genes. These signaling cascades recruit second messengers including NO, RTKs, GPCRs, and protein kinases including PI3K, serine-threonine kinase Akt, MAPK family members, and PKA and PKC. A typical example is the induction of antiapoptosis: ER associated MAPK pathway induce rapid phosphorylation of the adaptor proteins, Src and SHC, resulting in a SHC-GRB2-SOS complex formation; this leads to the subsequent activation of Ras, Raf, and MAPKs, including ERK1/2, JNK, and p38. They are then translocated to the nucleus and participate in gene transcription. (Courtesy of Hussam Al-Humadi, MD)
Most of the coregulators of the activator protein-2 (AP-2) (i.e., RIP 140, TIF-2, SRC-1, and SHP) interact equally well with ERα and ERβ [53–55], while others, such as the TRAP 220 coregulator, show significant differences in the interactions with ERα and ERβ [56, 57]; corepressors preferentially associate with ER antagonist [58–60]. Since distinct ERα and ERβ ligands are known to effect preferential recruitment of different coactivators [61, 62], the selective receptor/coactivator interactions represent an efficient system through which the pleiotropic effects of ER ligands might be mediated and are likely further determined by tissue-specific patterns of posttranslational modification of coactivators [63].
In summary, transcriptional activity of ERs is strongly influenced by ligands and the conformational changes induced upon ligand binding of ERα or ERβ, the formation of dimers (i.e., ERα/α and ERβ/β homodimers or ERα/β heterodimers), and the cofactor recruitment including interaction with chromatin. The co-expression of ERα and ERβ in different tissues results in a heterogeneous pool of prο-proliferative ERα/α and antiproliferative ERβ/β homodimers and in ERα–ERβ heterodimers that have different biologic effects than the homodimers [64–69]. Thus, according to receptor subtype and the cell type [70, 71], gene activation or repression can happen [72–75].
Peptide growth factors are also capable of eliciting estrogen receptor-dependent activation of an ERE of DNA; ER-dependent transcriptional activation can also be elicited by both protein kinase A and protein kinase C pathways [76–79]. In some cases, genomic effects are effectuated through protein–protein interactions [80]. In the absence of an ERE (around one third of the genes in humans that are regulated by ERs do not contain ERE-like sequences [81]), the ER–ligand complexes can bind to activator protein-1 (AP-1, a transcription factor which is a heterodimeric protein composed of proteins belonging to thec-Fos, c-Jun, ATF, and JDP families) or interact with transcription factors NF-κB (nuclear factor-κβ), and the SP (specific protein-1) to influence gene transcription [70, 72, 82, 83].
All abovementioned genomic mechanisms of action of estrogens mediated through the ERs activation upon ligand binding take time to be effectuated, but estrogens exert rapid and transient membrane-initiated effects as well; these effects occur within seconds or minutes, are known to involve several signalling cascades, and may also influence gene transcription in the nucleus (Fig. 1.2) The second messenger signalling events include stimulation of adenylate cyclase and production of cAMP [76, 84], mobilization of intracellular calcium[85], stimulation of PI3K and PKB [86, 87], and activation of MAPK pathway of Src with consequent activation of the extracellular-regulated kinases Erk1 and Erk2 [88–92].
Although most of the rapid effects of estrogens are believed to be mediated through activation of nuclear ERS (ERα and ERβ) localized near the cell surface (a small amount, approximately 2 %, of either ERα or ERβ can associate with the cell membrane [93]), novel membrane ERs (mERs) have been identified in a number of tissues. Membrane receptors are located in caveolae (specialized membrane invaginations enriched in the scaffold protein caveolin-1) at the membrane [94] and can bind to caveolin-1, G proteins, PI3 kinases, Src kinase, Ras, etc. (Fig. 1.2) [95–101].
An indirect induction of nongenomic effects can indirectly activate the gene transcription (i.e., the activation of a nuclear ER through phosphorylation by both Src/Erk and PI3K signalling in the absence of a ligand), and thus, the modulation of the functions of ERs by nongenomic actions of estrogens may augment the classical mechanism of ER action.
The possible convergence of genomic and nongenomic actions on target genes is an attractive mechanism by which ERs can finely regulate gene expression [78, 102]. It has been suggested that some of the responses to selective estrogen receptor modulators (SERMs) are mediated through nongenomic actions, which subsequently lead to genomic responses [103].
Similarly to estradiol, EDCs with estrogenic activity interfere with the functioning of the complex endocrine system acting through the ERα and ERβ receptor-mediated mechanism. The EDC receptor–ligand complex results in conformational changes and may activate EREs in a different way than the natural estrogen and thus influence the response in a qualitative and quantitative way, i.e., by mimicking the action of naturally produced hormones, they set off similar chemical reactions in the body, and by blocking the receptors in cells, they prevent the action of normal hormones, sometimes in a nonreversible manner [104]. Xenoestrogens seem to have equal binding affinity either to ERα or to ERβ [105] [the final effect in a specific tissue seems to be regulated by the ratio of the two ER isoforms (ERα, ERβ)], and they can selectively activate or repress estrogen-responsive genes in a different mode than the natural estrogens [104].
EDCs can bind either to estrogen receptors acting as estrogens or antiestrogens [106] or to androgen receptors acting as androgens or antiandrogens [106], but some EDCs can activate both receptors (bisphenol A binds to ER and acts through the genomic pathway [107], the pesticide o,p′-DDT also binds and activates the ER [108, 109], while the p,p′-DDE (the DDT metabolite) acts as an androgen antagonist but also as a weak estrogen receptor agonist compared to o,p′-DDT [110, 111]). The chemical structure of these compounds does not predict their activities, and small changes can alter affinity for the receptor; a typical example is the 5-carbon DPP that has 3-fold increased antiandrogenic potency compared to 4-carbon DBP. It seems that the structure function relationship is very complex.
Except for the genomic effects, some xenoestrogens, such as endosulfan, nonylphenol, and o,p′-DDE, induce rapid nongenomic effects by binding to membrane estrogen receptors (mERα, mERβ, and GPR30); the consequent activation or inhibition of several kinases including Erk1/Erk2, PI3K, MAPK, PKC, and PKA kinases triggers signal cascades. Activation of Ca2+ and K+ channels, intracellular Ca2+ concentration signals, cell proliferation, and apoptosis are effectuated through these pathways in several cell types [112–115].
Nongenomic effects of xenoestrogens have been observed in many cell types including pituitary cancer cells, breast cancer cells, cells of the immune system, neuronal cells, and bone tissues [109, 115–119].
The different classes of EDCs show a diversity of effect patterns and a distinct effect profile: they can induce genomic (nuclear) and nongenomic (extranuclear) effects or both of these effects, independently of each other and thus in conjunction with the activation or inhibition of other signalling pathways (e.g., PI3K); this might lead to an indirect promotion of the transcriptional activity of the ER [107, 120]. The substantial differences in the way they exert their effects through steroid receptors and the ability of compounds to activate either or both pathways are mostly influenced by their chemical structure: as an example, the long-carbon-side-chain alkylphenols show weak estrogenic activity in genomic assays and the shorter-side-chain versions even weaker, while the short- or long-carbon-chain variants show quite robust nongenomic activities [121–123].
Some EDCs, such as the chlorinated hydrocarbon β-HCH, although not binding to ER, are capable of activating ER target genes in a pattern very similar to the profile observed with estrogens. In order to explain these type of estrogenic effects of some compounds, Norman et al. [124] have proposed for the ERS the “two ligand-binding domains,” the “classical” and the “alternative” ligand-binding domain, responsible for the prolonged genomic events and the rapid nongenomic signalling, respectively. According to this theory, a ligand binds to the binding site it better fits; the conformation of a membrane-bound receptor favors binding to alternative site. Thus, compounds such as β-HCH and p,p′-DDE might have different affinities for the two proposed binding domains of the ER: p,p′-DDE fails to interact with the “alternative” domain in the membrane ER and consequently nongenomic effects cannot happen; on the contrary, β-HCH shows affinity to the “alternative” domain and thereby a sustained activation of Src/Ras/Erk pathway that may also lead to the strong activation of a number of other signalling cascades (such as PI3K and PKC), in addition to Src/Ras/Erk pathway [125–127].
As many estrogen agonists and antagonists, xenoestrogens have the ability to selectively bind membrane estrogen receptor [selective membrane receptor modulators (SmERMs)].
Although many of the EDCs’ effects are through binding to estrogen receptors, acting as agonists or antagonists, some of them bind to androgen or aryl hydrocarbon receptor (AhR) [128] (Fig. 1.3). The aryl hydrocarbon receptor (AhR) is a member of the basic helix–loop–helix Per (Period)–ARNT (aryl hydrocarbon nuclear translocator)–SIM (single-minded) (bHLH-PAS) family [129]. Upon ligand binding, the AhR translocates from the cytoplasm to the nucleus where it binds its dimerization partner ARNT. The activated AhR/ARNT heterodimer complex binds to xenobiotic response elements (XREs) and activates the expression of AhR target genes, such as cytochrome P4501A1 (CYP1A1) and CYP1B1 [130]. As shown in AhR-null animals, the AhR mediates most, if not all, of the toxic effects of 2,3,7,8-tetrachlorodibenzo-[p]-dioxin (TCDD) [131]. Although its physiological role is unknown, the AhR has been shown to be important in liver development and female reproduction [132]. Since AhR is a receptor for many ligands, striking synergistic effects can be anticipated.
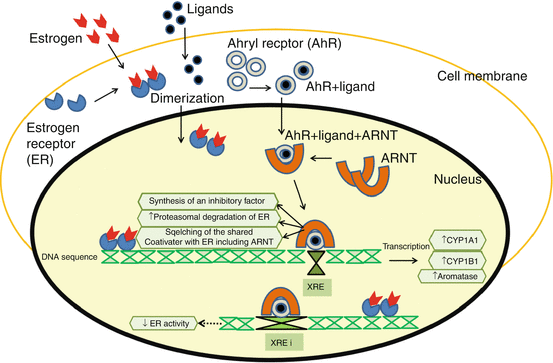

Fig. 1.3
Activation of ER and Ahryl receptor including relevant proposed mechanisms of crosstalk between their signaling pathways from the step of heterodimerization for Ahryl with ARNT and homodimerization for ER. AhR has been reported to inhibit ER activity through a combination of several different mechanisms: direct inhibition by the activated AhR/ARNT heterodimer through binding to inhibitory xenoestrogen receptor (iXRE) present in ER target genes; squelching of shared coactivators, including ARNT; synthesis of an unknown inhibitory protein; increased proteasomal degradation of ER; and altered estrogen synthesis/metabolism through increase in aromatase, cytochromeP450 1A1 and 1B1 expression. ((Courtesy of Hussam Al-Humadi, MD)
Inhibitory crosstalk between the AhR and ER signalling was suggested by early experiments examining the long-term effects of TCDD treatment in Sprague Dawley rats [133]. The first observations, that the incidences of both mammary and uterine tumors decreased in female rats [133] after exposure to dioxins, were supported by other reports demonstrating that TCDD inhibits the formation of 7,2-dimethylbenz[a]anthracene (DMBA)-induced mammary tumors [134].
The precise molecular mechanisms for this crosstalk are unclear and may be a combination of several different mechanisms. Several studies have reported that the activated AhR inhibits the expression of E2-induced genes [135, 136], causes decrease of the levels of nuclear ER, and is involved in mediating the antiestrogenic responses in target cells/organs [137–140]. Thus, it is possible that the primary antiestrogenic action of TCDD is to downregulate expression of the ER gene, thereby reducing cellular ER levels.
EDCs can disrupt the homeostasis of a multicellular tissue by inhibiting the gap junctional communication (the intact communication between adjacent p cells through the connexin-lined gap junctions (Gjs) is a requisite for maintaining homeostasis) [141]. Furthermore, they can dysregulate the effects of effects in an indirect way by disrupting hormone levels; they can inhibit or activate the expression of the P450 enzymes, with consequent alterations in the synthesis, transport, metabolism, and excretion of endogenous hormones, i.e., inhibition of enzymatic activity of the P450 family members CYP19 and CYP3A1, which convert testosterone to estradiol, decreases the hormone synthesis.
EDCs may act at the cellular and molecular levels, binding to both steroid and aryl hydrocarbon receptors exhibiting both dependent and independent receptor modulations of specific gene transcriptional elements [142–144]. As a result, xenoestrogens have the potential to variably modulate cell proliferation, cell cycle progression, apoptosis, and cytokine production in much the same way as 17-β-estradiol does [145, 146].
1.2.2 Cytochrome P (CYP) Induction
Exposure to EDCs can interfere with the induction of the phase I enzymes of the cytochrome P450 family. An example is CYP1A that is inducible by several classes of EDCs including the halogenated aromatic hydrocarbons (HAHs), the polycyclic aromatic hydrocarbons (PAHs), and the dioxins [2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)] [147]. In the presence of a ligand, the AhR/ARNT heterodimer binds to the xenoestrogen responsive elements in the promoter of the CYP1A gene [147, 148] while at the same time the induction of many other genes happens [149, 150] (Fig. 1.3). CYP induction that occurs by a process involving de novo RNA and protein synthesis [151] has been shown to be important in the metabolism of xenoestrogens and the generation of reactive genotoxic metabolites [152, 153]. By this procedure, weakly active procarcinogens can be transformed into electrophilic intermediate metabolites capable of reacting with DNA, raising the risk of developing cancer; a typical example is the case of breast cancer in which the induction of the ile/val and val/val alleles of the cytochrome P450 1A1 gene, under certain circumstances, may result in increased risk [154].
1.3 Effects
The endocrine system regulates complex functions and thus hormone dysregulation results in a wide array of effects. Endogenous estrogens (17-β-estradiol, estrone, and estriol) are not only regulating the development, maintenance, and function of the reproductive system in both sexes [155–157] but they also exert important biologic effects in many tissues and organs: they affect cognition and behavior in the central nervous system; they are involved in the cardiovascular health, have a significant impact on cell-mediated and humoral immune and autoimmune responses, and play a role in adipocyte development and function as well as in bone growth and epiphyseal plate closure in both sexes [158–163]. They are implicated in the development or progression of numerous diseases including breast and colon cancer, osteoporosis, cardiovascular and neurodegenerative diseases, endometriosis, and obesity [164–167].
In relation to estrogens, EDCs can have direct toxic effects on an endocrine gland and indirect endocrine toxicity to non-endocrine organs [168]. The effects of EDCs in wildlife have been documented by many studies; the most prominent include masculinization in snails, hermaphroditism in fish, distorted sex organ development and function in reptiles (alligators and turtles), abnormal nesting behavior and induced eggshell thinning in birds, and disturbed reproduction and immune functions in grey seals [169, 170].
In humans, xenoestrogens have been mainly accused for cancer, neurological and immunological effects, reproduction failure, and osteoporosis, but data are still contradictory. The link between man-made chemicals and adverse effects that usually appear as domino effect is not quite clear. The causative role of chemical substances in diseases and abnormalities related to endocrine substances has not been well documented in human health, even though various articles have appeared describing the growing evidence that man-made chemicals are causing adverse effects in both humans and wildlife by poisoning the hormone system. The fact that adverse effects in animals do not predict the same results in humans and many effects appear to be species specific makes the issue even more difficult; i.e., exposure to phthalates causes suppression of testosterone in rat and stimulation of testosterone in the mouse, while no clear effects have been demonstrated in humans [171, 172]. The accidents in Seveso and in Taiwan (Yu-cheng disease) gave a lot of information about the connection of EDCs and human health [171, 173], but in order to establish a clear cause–effect relationship, geographical, social, diet, lifestyle and inter-population variations should be taken into consideration.
The diversity of mechanisms, the complexities and interactions of endocrine signalling mechanisms, the variety of possible end points, and the broad range of chemicals possibly involved in the adverse effects in humans and wildlife make the issue difficult to understand in its various aspects; thus, before the hypothesis becomes a certainty, it might take a lot of time.
Some of the effects associated to the exposure to xenoestrogens are presented in the following sections.
1.3.1 Effects on Female Genital System
The fundamental role of estrogens in females during puberty and reproductive cycling is well known [174, 175]. Estradiol exerts complex effects on gonadotropin-releasing hormone (GnRH) neuronal function including long-term genomic effects through binding to ERα and/or ERβ subtypes and rapid nongenomic effects such as glutamate-induced currents in hippocampal neurons and second messenger cascades in hippocampal or hypothalamic neurons [85, 176–179].
Xenoestrogens seem also to affect the physiology of the genital system in women, since in epidemiological studies, they have been associated with menstrual disorders, abnormal ovulations, endometriosis, and spontaneous abortions.
Similarly to estrogens, EDCs, in particular o,op′-DDT, can modulate the GnRH secretion in vitro in the immature female hypothalamus through both slow and rapid effects; in these effects, glutamate plays an important role with the participation of genomic and nongenomic pathways involving several receptors (ERs, AHR, and AMPA) and intracellular kinases (A, C, and MAPK) [180]. The early onset of puberty observed after exposure to EDCs is probably connected to GnRH stimulation [181–183].
Epidemiological studies have shown that women who consumed fish from Lake Ontario, polluted with organic pollutants, had reduced cell cycle length, while endocrine dysfunction was found in women exposed to pentachlorophenol [184–186]. Endometriosis represents a common gynecological condition reaching 5–15 % of childbearing-age women and up to 3–5 % of postmenopausal women. Although endometriosis has developed in rats [187, 188] and in monkeys [189] after exposure to dioxins, the data in women are still conflicting; thus, in some studies, EDCs acting through an Ah receptor mechanism [190] have been associated with endometriosis, while in others, there is no evidence of such association [191, 192]. Early menopause has also been referred in women exposed to perfluorocarbons [193].
The concentrations of estrogens in the plasma seem an important factor for the manifestation of the effects after exposure to chemicals with estrogenic activity; thus, menopaused women under hormone replacement therapies are less vulnerable than those who do not take estrogens, while prepubertal girls are more vulnerable compared to older ones [194]. Several of the effects of the estrogenic compounds are also due to the alterations in the aromatase activity and thus changes in the estrogen concentrations [195, 196].
Reproduction problems have also been connected to the extensive use of EDCs. A decrease in fertilization rates after IVF has been found in couples in which the husband was exposed to EDCs (pesticides) [197]. Furthermore, exposure to organochlorides has been associated in some studies with spontaneous abortions [198–200], but according to other reports, pregnancy outcome was not affected [201].
A causal relationship between malformations in the urogenital system and exposure to EDCs had been strongly suggested by the “feminization” of the population in some areas with high discharge of these compounds [202]. The prevalence of birth of more girls than boys from young fathers in the Seveso accident in 1976, a fact observed and in many industrial countries, has been connected with exposure to dioxins [203, 204]. Structural and functional defects in the female reproductive tract have been observed after exposure to diethylstilbestrol and other xenoestrogens such as the pesticide methoxychlor; these compounds have been shown to disrupt the development of the female reproductive tract by altering HOX gene expression (HOX gene determines the differential developmental identity of the Müllerian duct) [205, 206].
Estrogens have also been implicated in endometrial cancer through involvement of G protein-coupled estrogen receptor GPR30 and consequent activation of the PKC pathway [207]. Another possible mechanism is that the 2-OH and 4-OH estrogen metabolites can be further oxidized to semiquinones and quinones, which can form bulky DNA adducts and initiate carcinogenesis (Fig. 1.4).
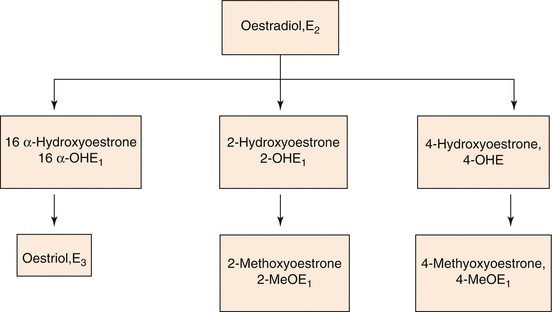

Fig. 1.4
Metabolic products of Oestradiol (E2)
1.3.2 Effects on Male Genital System
Estrogens have a fundamental role in male genital system and consequently in male fertility. Estrogen receptors have been found in mature and fetal testis and in epididymis as well, indicating their importance in regulation of spermatogenesis: ERα is mainly localized in Leydig cells, and ERβ is mainly localized in Leydig and most germ cells, while aromatase, the enzyme that converts testosterone or androstenedione to estradiol, is found in Leydig cells, Sertoli cells, and germ cells [208–210]. Estrogens induce also both proliferative and antiproliferative effects; some of these effects are mediated through binding to ERβ, and consequent downregulation of the androgen receptor ending in induction of apoptotic mechanisms [211].
In view of the important role of estrogens in the male genital system, and since men are routinely exposed to estrogen-like compounds, various changes in male physiology and fertility have been attributed to these chemicals [212, 213].
Studies in wildlife have shown that some of the EDCs, as the metabolite of p,p′ DDE, exhibit antiandrogenic activity; exposure of the alligators in Lake Apopka to DDT showed a progressive decline in the population and abnormal genital structure [214]. Impaired fertility was also shown in experimental animals after exposure to lindane or PCBs [215, 216].
Prenatal exposure of experimental animals to DES resulted in increased incidence of cryptorchidism, urethral abnormalities, testicular hypoplasia, poor semen quality, rete testes adenocarcinoma, and cell hyperplasia [217–219]. Similarly, DES-exposed males have shown pseudohermaphroditism, genital malformations (small testes, testicular abnormalities, microphallus), and reduced semen quality [220]. The genital system seems to be vulnerable when the exposure to estrogenic chemical compounds happens only at a critical period of neonatal life [221]. The effects of some xenoestrogens on sperm quality seem to be effectuated through a nongenomic pathway [222].
Epidemiological studies in various European countries including France, Sweden, Scotland, and Greece have shown a progressive decline in sperm analysis attributed to exposure to compounds with estrogenic activity (i.e., the pesticide dibrochloropropane, DBCP) [223–227]. Reduced sperm concentration and motility was found in higher prevalence in semirural and agricultural areas compared to more urban areas. Workers exposed to dioxin had decreased serum testosterone and increased LH [228]. The increased incidence of hypospadias and cryptorchidism in some countries has also been associated with prenatal or paternal exposure to EDCs [229, 230].
Increased incidence of testicular cancer, a malignancy more common in young men, has been observed in many countries [231–233]. Etiologic agents or conditions for testicular cancer include, among others, exposure to pesticides and field exposure to hydrocarbons and polyvinyl chloride, but many authors have linked the increased incidence with embryonal exposure to EDCs [234–237]. The mothers of men with testicular cancer showed higher concentrations estrogenic compounds [238].
Although the data are not conclusive and sometimes are contradictory, the most possible etiology for the testicular dysgenesis syndrome (TDS) (a disorder of the male reproductive function including decrease of sperm count and increased incidence of testicular cancer and hypospadias and cryptorchidism), which has shown an increase in a small time period, seems to be rather an environmental and not a genetic factor [234, 238, 239].
According to recent studies, xenoestrogens can affect male fertility through a transgenerational epigenetic action on male reproduction system; thus, a transient in utero exposure to a xenoestrogen influences the embryonic testis transcriptome and through epigenetic effects results in abnormal germ cell differentiation that subsequently influences male fertility [240].
1.3.3 Breast Cancer
Estrogens are hormones with genotoxic potential and may act as carcinogens at non-physiological doses. Their carcinogenic effects seem to be independent of ERs, although ERs could play a role in the early stages of cell transformation, invasion, and tumorigenesis [241]. Increased expression of specific proteins and induction of oxidants and aldehydes ending to and cause lipid peroxidation are among the mechanisms estrogens cause cancer. Furthermore, several oncogenes have been shown to encode the growth factors and their receptors that are activated by estrogens (close relationship), i.e., c-erb-1 oncogene encodes the EGF-r (transmembrane receptor protein, whose extracellular domain is overexpressed in many cancers) [242, 243]. Estrogenic compounds, given their capacity to perturb normal hormonal actions, have been associated to the development of hormone-dependent cancers, such as breast and endometrial cancers and testicular cancer [244].
Similarly to estrogens, many EDCs induce an increased activity in a series of genes in which transcription products are growth factors involved in the carcinogenic process, i.e., EGF, TGFa, IGF1, and their receptors; this fact makes proliferation uncontrollable [245]. The first well-studied case of the association between cancer and estrogenic compounds is the example of diethylstilboestrol (DES), in which the daughters of the pregnant women that had been exposed to DES developed a clear cell adenocarcinoma of the vagina and the cervix [246].
Cancers are traditionally presumed to occur without a threshold; as a consequence, any dose of a carcinogen is associated with an increased risk.
Estrogens have been implicated in the development of breast cancer. In the USA, each year 44,000 women die of breast cancer, making it the leading cause of cancer deaths among American women that do not smoke and among those aged 40–55 years. Increased incidence of breast cancer in all age groups has been shown in various countries. The elevated incidence of breast cancer has been associated with prolonged and cumulative exposure to high levels of estrogens, i.e., early onset of menarche and late menopause, obesity, and hormonal replacement therapy (HRT) [247–251]. Decreased levels of SHBG (sex hormone-binding globulin) have also been associated with increased incidence of breast cancer due to increased levels of free estrogens. Estrogen metabolites have also been implicated in the increased incidence of breast cancer. A shift of the normal metabolic pathways of estrogens to alternative routes may involve carcinogenic metabolites; thus, if instead of the activation towards the 2-hydroxyestrone metabolite (2OHE1) production that acts as weak antiestrogen and is not carcinogenic, a shift to 16a OHE1 pathway occurs, it gives rise to fully potent active estrogens (Fig. 1.4). The 16a OHE1 pathway metabolites are genotoxic and carcinogenic; they circulate in very small amounts but they remain unbound due to their low affinity for SHBG and thus are free to covalently bind to the nuclear ER and to form stable adduct interacting with nuclear histone proteins [252].
ERα, the important receptor for breast development [253], is the mammary mediator of estrogenic effects in breast cancer (both in cell cultures and in breast tissue) [254]. Several sequence variations or single-nucleotide polymorphisms (SNPs) in the ERα gene (ER1) have been associated with increased risk of cancer [255].
Reduced binding of estradiol to SHBG may increase risk for breast cancer development [256].
Increased levels of androgens have also been implicated in breast tumor development mainly serving as substrates for estrogen [257].
In a number of epidemiological and cross-sectional studies, EDCs, such as PCBs, DDE, and dieldrin, are included among the risk factors for breast cancer [258, 259]. Increased risk for breast cancer has been shown in countries with medium or high levels of exposure to various EDCs (i.e., triazine pesticides) [260]; a positive correlation between organochloride concentration in adipose tissue and the development of breast cancer [261, 262] has been shown as well. A possible connection between the levels of some pesticides acting as xenoestrogens in breast milk and adipose tissue cannot be excluded [261, 263]. The fact that in Seveso a decrease incidence in breast cancer was reported shows the complexity of the whole issue [264].
Epidemiological data have linked early-life TCDD exposure and diets high in fat to increased risk for breast cancer in humans; high-fat diet has been shown to increase sensitivity to maternal TCDD exposure, resulting in increased breast cancer incidence, by changing metabolism capability [265]. Even a single exposure to a xenoestrogen, if it happens during a critical period of life, may alter epithelial differentiation and lead to increased multiplicity of tumors or decreased latency of tumor formation [266]. P 53 mutations may also be implicated in the susceptibility to EDCs for breast cancer development [267].
The carcinogenic or noncarcinogenic effects of estrogens have been associated to the initiation of estrogen metabolism by cytochrome P450 enzymes CYP1B1, CYP1A1, and CYP1A2 [147, 268–270]. Estrogenic compounds like dioxins, PCDFs, and some PCBs, acting in a similar way, induce CYP1A1, CYP1A2, and CYP1B1 gene expression by serving as aryl hydrocarbon receptor (AhR) agonists; CYP1A1 and CYP1B1 catalyze hydroxylation of the A-ring of estradiol (E2) to form the catechol estrogen 2- or 4-hydroxylestradiol (2-OH-E2 or 4-OH-E2, respectively) (Fig. 1.4) [271].
The discrepancies between EDCs as risk factors and breast cancer found in various studies are probably due to the fact that in the breast cancer development a complex mixture of estrogenic chemicals is involved and not only one factor [272].
1.3.4 Obesity
Nowadays, obesity has risen dramatically not only in industrialized countries but also in poorer countries reaching epidemic proportions [273].
Estrogens through ERα and ERβ are also involved in the regulation of body fat distribution and metabolism [274–277]; ERβ has been shown to have anorectic effects mediated via the central nervous system [278], while disruption of ERα in the ventromedial nucleus of the hypothalamus leads to weight gain, increased visceral adiposity, hyperphagia, hyperglycemia, and impaired energy expenditure in female mice [279].
Studies in DES-exposed mice have indicated that the increase in body weight was associated with an increase in the percent of body fat [280, 281], and significant alterations in genes involved in fat distribution were altered [282]. Similarly to endogenous estrogens, a link between exposure to environmental chemicals (such as estrogenic chemicals, BPA, PCBs, DDE, and persistent organic pollutants and heavy metals) and the development of obesity has been shown in epidemiologic studies, in support of the findings in experimental animals and show [283–287].
Polychlorinated biphenyls (PCBs) and organochlorine pesticides have been associated with high levels of total serum lipids, fat mass, and BMI, while non-dioxin PCBs were shown to be inversely associated with BMI [288–291]. Moreover, prenatal and early-life PCB exposures have been associated with increased weight in boys and girls at puberty [292]. Other studies report a link between some persistent organic pollutants and increased body weight and diabetes [293].
1.3.5 Diabetes
Diabetes mellitus represents one of the most serious health problems worldwide with more than 177 million people suffer from it, and it is among the leading causes of death [294]. Estradiol seems to play an important role in energy balance, lipid metabolism, and glucose homeostasis [294–299].. Estradiol increases insulin biosynthesis and release in an ERα-dependent manner [300, 301], rapid nongenomic insulinotropic action on b cells is effectuated through ERβ [302], and both receptors (ERα and ERβ) can modulate GLUT4 expression in skeletal muscles of mice [303]. Similarly to E2, exposure to BPA and other persistent organic pollutants (POPs) like dioxins, furans, polychlorinated biphenyls (PCBs), or organochlorine pesticides, stored in white adipose tissue, have been strongly associated with type 2 diabetes and with most of the components of the metabolic syndrome; cardiovascular disease and liver enzyme abnormalities are established in several cross-sectional studies [290, 304–307]. BPA exposure disrupts pancreatic β-cell function and causes hyperinsulinemia [300] and mild insulin resistance, increases basal and insulin-stimulated glucose transport (due to an increased amount of GLUT4 glucose transporter) [308], stimulates adipogenesis [309, 310], and inhibits adiponectin release leading to increased risk for metabolic syndrome [311]. A significant relationship between BPA concentration in urine and type 2 diabetes has been found [304].
1.3.6 Neurologic Defects
The development of the central nervous system both in utero and during childhood is a continuous process in which many morphologic changes take place. Estrogens play an important role in neural development. Both ER receptors are highly expressed in the brain; ERα receptors are present in higher concentrations in the hippocampus, and ERβ receptors are present in higher concentrations in the basal forebrain and cerebral cortex. The neuroprotective effects of estrogens against neuronal cell death [312, 313] have been documented both in vitro and experimental animals: estrogens regulate the dopaminergic neurotransmission [314–316], promote the growth and survival of cholinergic neurons, and increase cholinergic activity, but they also have antioxidant and antiapoptotic effects [317–320]. The data from clinical studies in neurodegenerative diseases (Alzheimer’s disease and Parkinson disease) are inconsistent and even controversial; estrogens have been positively, negatively, or with no effect correlated with the onset and the severity of the diseases [319, 321–324]. Increased risk of dementia has been associated with lower lifetime endogenous estrogens [325, 326]. Male–female differences in the clinical and cognitive characteristics of several diseases have also extensively been discussed [327–332]. The protective effects have been connected to ERα rather than ERβ activation, through ER-dependent and ER-independent mechanisms or both are involved [313, 333].
Similarly to estrogens, EDCs act directly on CNS, and since the brain is a very sensitive target of steroid action, especially during development, EDC exposure might cause severe problems. Reproductive behavior, learning and memory, and other functions are permanently impaired after perinatal exposure of experimental animals [334]; male rats exposed to TCDD during the perinatal period have shown altered sexual differentiation in the brain, involving sexually dimorphic reproductive and nonreproductive neural end points. EDCs also affect neuronal synapse formation [335]. Many of the effects of the EDCs in CNS are mainly effectuated through the ERβ receptor, but the effects vary depending on the chemical compound, i.e., bisphenol A and methoxychlor affect the dopaminergic and noradrenergic systems in rodents [334, 336], but they are associated to sensory or cognitive deficits after exposure during the neurodevelopment period [337]. The effects of TCDDs though seem to be effectuated through the Ahr receptors found on GABAergic neurons (GABA and glutamate regulate learning and memory functions, stress responses, social behaviors, and mood [338, 339]).
Exposure of humans to EDCs has resulted in effects on behavior changes, learning problems, memory, attention deficit, and impairment of sensory and psychomotor development [340, 341]. Neurologic disorders and cognitive (impairment of memory and attention) and behavioral problems had been reported in young children whose mothers had consumed food contaminated with PCBs in addition to growth retardation [342, 343].
Exposure to PCBs has been associated with a memory deficit at 7 months and at 4 years of age, while up to 11 years of age, a negative association was reported between deficit of IQ (intelligence quotient) and PCBs’ concentrations index (the index was comprising maternal and cord serum and maternal milk concentrations). Other studies have reported a significant decrease in mental developmental index score as a function of maternal breast milk levels of PCBs at 2 weeks postpartum, which probably reflects maternal body burdens during pregnancy. Most of the studies showed a decreased IQ and poorer cognitive functioning in preschool children [344–346]. Verbal functions are longlasting, while visual–spatial functions, episodic memory, and sustained attention may be less sensitive to prenatal PCB exposure [347].
Some EDCs cross the placenta readily and the blood–brain barrier in the fetus; thus, exposure to these agents can impair mental and physical ability due to altered bioavailability. It is becoming clear that developmental exposure to EDCs and dioxin-like compounds can permanently impair neuroendocrine functions. Several studies, in various countries, have been conducted in order to establish the association between prenatal exposure to xenoestrogens and several aspects of psychomotor development [348].
The data are not conclusive and the discrepancies found between the clinical studies are probably due to the different methodologies used for the assessment of the neuropsychological problem and the parameter examined [349, 350]. Another possible reason is that isolated xenoestrogens do not reflect the effect of exposure to a mixture [351].
1.3.7 Immunologic Effects
The relationship between autoimmune system and endogenous estrogen levels is well established [352]. Estrogens mediate their effects via estrogen receptors (nuclear isoforms and/or membrane receptor) in different cell types of the immune system (B cell, T cells, dendritic/macrophages, monocytes) [353]. They regulate T cytokine gene expression via ER-mediated pathways, either directly through EREs or indirectly through interaction of ER with other transcription factors including NF-kB and AP-1 [354, 355]; NF-kB response elements have been found in the promoter of several cytokine genes like IL-6, IL-10, TNF-a IL-1β, IL-12, and IL-2 [356–358]. Thus, estrogens by acting via their receptors and their crosstalk with other transcription factors in immune cells and organs can modulate immunological parameters [359].
Exposure to various classes of EDCs (such as DES, TCDD, PCBs, organochlorides) has been shown to cause immunosuppression and potential disease susceptibility [360, 361] both in humans and animals; dolphins exposed to EDCs (DDT, PCBs) showed impaired immune function [362]; decreased immune function and increased incidence of infections has also been observed among affected people [363]. After the incidence of Japan in 1968 and in Taiwan 1979 from contaminated rice oil, increased incidence of rheumatoid arthritis had been found, while the patients affected by the Yusho disease suffered respiratory infections for a long time [364–366]. Perinatal exposure to estrogenic compounds (i.e., dioxin) has been associated with increased incidence of infections (respiratory infections, otitis) [367], lower white blood cell count during the first years of life; reduced thrombocytes have also been reported to dioxin exposure [368, 369]. Allergic asthma has been associated with phthalate exposure since they induce enhancement of mast cell degranulation and eosinophilic infiltration which are important parts in the early inflammation phase [370]. Exposure to EDCs has also been associated with increased prevalence of thyroid antibodies [371].
PCDDs and related compounds may be related to immune diseases, such as atopic dermatitis. The effects of these compounds on the immune system were very clearly shown on the babies of young Japanese after the oil accident [372]. But important questions of clinical relevance of real-life exposure and identification of molecular targets that can explain the interactions remain to be answered.
1.3.8 Effects on Bones
Estrogens regulate skeletal homeostasis in both men and women. They enhance osteoblast bone formation, and their deficiency has been associated with increased bone resorption and osteoporosis [373, 374]. Exposure to environmental chemicals that are able to disrupt the hormonal equilibrium might represent another risk factor for this disease [254].
Estrogen receptors ERα and ERβ have been found in both osteoclasts and osteoblasts. They are differentially expressed in the growth plate and mineralized bone, ERα is more highly expressed in cortical than in cancellous bone, and ERβ is most evident at cancellous than cortical sites, suggesting that they may have different functions [375–377]. The role of ERα is clearer compared to ERβ in bone formation [378].
The effect of estrogens in bone seems to be age and sex specific [379]. The importance of estrogens in males has been well documented from the fact that a loss of function mutation in ERα gene in a man has been connected with osteopenia [380].
In view of the important role of estrogen deficiency in osteoporosis, EDCs, since they interact with the ERs modulating the estrogen signalling pathway and altering estrogen metabolism, have been implicated in the pathogenesis of osteoporosis. Polychlorinated biphenyls, β-hexachlorocyclohexane, and 2,3,7,8-tetrachloro-dibenzo-p-dioxin are among the compounds that have been associated with osteoporosis [381, 382], but the relation between organochlorine exposure and bone quality and osteoporosis is not clear; thus, further studies are needed [383].
1.3.9 Exposure In Utero and During Lactation
The exposure of the embryo to EDCs has been a major concern. The exposure to these chemicals begins from the first days of the in utero life since the placenta easily permits substances with low molecular weight to enter the fetal circulation, suggesting that these compounds can affect organogenesis [384]. Since these chemicals are lipophilic, they tend to accumulate in the adipose tissue of the pregnant women [385] and from there to be transferred to fetuses and infants through the placenta and breastfeeding. Since EDCs cross the placenta, the embryo is exposed to these chemicals and their metabolites; the neonate is further exposed to relatively high EDCs concentrations found in milk.
Even in the absence of epidemiological studies, concern over adverse effects of xenoestrogens is warranted given the unique vulnerability of the developing fetus and child [386]; placenta does not protect the embryo, and the embryo and young children lack the protective mechanisms an adult disposes, including liver metabolism, detoxifying mechanisms, and blood–brain barrier. Maternal exposure to phthalates has been connected to sex steroid hormone status in fetal and newborn stages. Prenatal exposure to DES has been connected with adverse effects on the reproductive tract both in male and female offspring, including pseudohermaphroditism, genital malformations, and a reduced semen quality [218, 387]. Prenatal BPA exposure has the potential to alter neurodevelopmental, reproductive, and metabolic end points throughout the life span [388–390] at low doses, while at high doses fetal viability is compromised [391].
Although many efforts have been done to establish the lower threshold doses for toxicity, the issue has not been resolved for many products [392]; since there is no threshold in endocrine systems and no safe doses that exist. Actually an effect can be observed in lower doses, while in higher doses little or no effect is shown [393]. One of the reasons the data so far are conflicting is that many methods used lack sensitivity and precision [394]. Furthermore, it is important to take into consideration that the effects on the embryo also depend upon the developmental stage when the exposure happens and they are gender specific. Prenatal and perinatal stages are the most susceptible to the vulnerable effects, but the particular window of exposure during prenatal life makes the whole issue more complicated and the conclusive decisions about the harmful effects difficult. In this context, DDT use has been shown to increase preterm births, which is a major contributor to infant mortality [395].
EDCs can modify gene transcription disrupting the normal signalling systems that determine fetal development, and according to Colborn, they can impose a life sentence on the embryo [396]. Such an example could be considered the higher risk of overweight and obesity later in life that is associated with exposure to EDCs during development.
Further exposure of the neonate is with lactation. Maternal adipose tissue is catabolized and mobilized during lactation to provide 60 % of the fats in milk fat [397]. The adipose tissue catabolism results in the release of persistent EDCs that have been accumulated over the years (i.e., PCDs, DDT) to breast milk. It is interesting that the higher concentrations of the compounds in breast milk are consumed by the first child because the mother’s fat stores are depleted with each subsequent child [398]. Human infants are exposed by breastfeeding, on a bodyweight basis, to doses of xenoestrogens that exceed the doses of adults by at least two orders of magnitude. In a group of breastfed children exposed to a PCB home environment, it was shown that the PCB concentrations were markedly increased with the duration of breastfeeding and were about five times higher than in the non-breastfed children [399].
An analysis of human milk revealed increased concentrations of several cosmetic chemicals (i.e., UV filters, synthetic musks, parabens); their concentrations were correlated to the frequency of use of the cosmetics [400]. Human breast milk levels of polybrominated organic compounds have increased 60-fold in the past 30 years and doubled in the last 5 years [401].
In view of the persistency of these chemicals, the ideal solution should be to place the mother and the infant in a protected environment, with no contamination of air, water, and food! But the practical solution for a pregnant and lactating woman should be to avoid consuming contaminated fish, such as fish from freshwater containing PCBS, and minimize exposure to products containing EDCs (cleaning products, paints, etc.). Single products may not be so hazardous but mixtures are [402].
The Greater Boston Physicians for Social Responsibility created a site for lactating women, where one can find information about the contaminated products with EDCs (http://www.igc.org/psr/breastfeeding.htm).
1.3.10 Risk Assessment
It is still not clear what the relationship between observed or assumed effects in humans and wildlife and exposure to man-made estrogenic compounds is; apart from the uses they are designed for, they may have unforeseen adverse effects or synergistic effects.
To make a prediction on human health consequences of the exposure to a range of substances which are suspected of interfering with the endocrine system (i.e., a risk assessment) is a very complex procedure since the following steps are important: identification of the substance, identification of the dose–response relationship, identification of a threshold dose which protects human health, quantification, and qualification of the adverse health effects. Thus, to predict a risk is a time-consuming procedure that needs knowledge of the mechanism of action, identification of the exposed population, and knowledge of the heterogeneity of the population, i.e., genetic predispositions, age and gender, diet, work exposure, and special conditions, such as pregnancy and lactation, route which exposures might occur and estimation of the magnitude, and duration and timing of the doses that people might receive as a result of the exposure. Together these studies indicate variable sensitivity to disruption by environmental chemicals during the developmental period and underscore the complexity of the mechanisms involved in their effects.
In order to examine the acute and chronic toxicity, carcinogenicity, genotoxicity, and effect on reproduction and development (organogenesis and fetal period) caused by EDCs, various pharmacological and toxicological tests have been developed [403].
A major problem in the case of EDCs is that one cannot make accurate predictions about the toxicity of xenoestrogens because of the absence of unambiguous dose–response relationships. Dose–response relationships are known for a number of individual EDCs; they present not a monotonic but a nontraditional dose–response curves such as an inverted “U” or even multiple “U”-shaped curves of their effects. Some effects are dose related, others dose independent or inversed according to the dose; some effects are reversible and others are not [404]. Due to the fact that the threshold model does not exist in the endocrine system, higher doses results are not predictive for lower doses effects [405], i.e., no dose effect has been found in dioxin that caused clear cell adenocarcinoma [406], while in the case of bisphenol A, very small doses have been shown to cause sex reversal in turtles [407]. In Belgium, the dioxin crisis was caused by only roughly 1 g of dioxin contained in 25 l of old PCB transformer oil [12, 13]. Furthermore, the fact that effects of many chemicals across species differ, the dose effect experiments in animals do not permit safe conclusions about the adverse effects in humans.
In view of the exposure to mixtures, the whole procedure becomes even more complex. Little is known about the interactive effects of mixture; the consequences on human health are multifactorial depending upon the concentration (ppm) of a certain compound in the product: i.e., in cosmetics, the volume of cosmetic used (ml) per application, times of applications per day, and rate of absorbance depending upon route of administration should be determined.
Lower observed effect levels (LOEL) and no effect levels (NOEL) are two of the indexes used, but for many EDCs, the current tests do not provide evidence for the existence of a NOAEL function. Synergistic or antagonistic effects are for the most part unknown; compared to prescribed medication, a medication’s license can be withdrawn upon reports on adverse effects, while it is very difficult to revoke the license of a non-pharmaceutical chemical because the data will be partially confounded by the mixture problem and means of effect is almost always lacking.
A major problem is that the exposures to EDCs are involuntary, often chronic in duration; nontoxic effects are known a priori, and the appearance of a new illness or pathology is documented only after high-dose disaster happens [408]. Then, while acute, subacute, and chronic exposure and reproductive and genotoxicity tests in animals are routine for pharmaceuticals for the majority of the chemicals, this does not apply. In general, any chemical with a production level of <1,000 tonnes/year in a country will require little testing. Thus, a considerable amount of research is still required to ascertain the scope and the seriousness of endocrine disruption, including confirmation of epidemiological studies.
The exposure levels that could have deleterious health effects are somewhat difficult to determine and are actively debated [409, 410], but a most critical point is the concentrations at the target organs are most critical. Thus, data on breakdown, excretion, and bioaccumulation are very essential.
Newer methods have been developed in order to measure the amount of EDCs in biological fluids and tissues (i.e., exposure to the phthalates in perfumes and their concentration in hair) [411, 412]. In a recent study, by use of a bioassay, the exposure to EDCs, estimated in total 17β-estradiol equivalents (EEQs), was found increased in occupational exposure to pesticides, disinfectants, and exhaust fumes [413].
Recently, a scalable and statistical method has been developed, in an effort to predict known and novel associations of several chemicals with prostate, lung, and breast cancers, using publicly available data (e.g., on estradiol and bisphenol) [414].
1.4 Importance of Identification of Compounds with Estrogenic Activity
The majority of the EDCs are compounds structurally unrelated, and the prediction of the estrogenic activity is very difficult, and only in a very small number of chemicals can be done. Thus, in view of the continuously increasing number of these compounds, governmental agencies were forced to examine the whole issue [415] setting as first target the identification of the compounds. Several screening tests have been developed; the principal requirement is to assess the potential of these compounds to interact with the endocrine system of man and wildlife in order to anticipate adverse effects and then to elucidate the mechanism of action. By the in vitro screening methods, the affinity to the nuclear ER (α, β) was evaluated [416–418]. From 58,000 synthetic compounds that were checked in 2002, including synthetic estrogens, natural products, several plasticizers, commercial chemicals, and impurities, 6,903 were found to dispose weak estrogenic activity (at least 1,000-fold less compared to E2) [419]. Among the bioassays, the E-SCREEN assay is a simple, fast, reproducible, reliable, and quite sensitive assay [109]; it has allowed the identification not only of the chemicals with estrogenic activity but their discrimination into estrogen full and partial agonist and antagonist compounds by measuring the cell proliferation on cell lines as well. Other assays that have been used are the binding receptor assay [109, 420], the cell proliferation assay [421], and the gene expression assay [422], but none of them can distinguish between agonist and antagonist. Although useful, in vitro assays suffer from problems associated with the absence of effective means to metabolize chemicals. Thus, the big problem is that EDCs must be evaluated in intact organisms; in vitro assays are of value just for evaluation of mechanisms of action or prescreening chemicals for potential endocrine-disrupting properties and for setting priorities for in-depth in vivo testing. Big efforts have been made in the USA, EU, Japan, and OECD in establishing appropriate tests and to harmonize the strategy efficiently. A two-tiered program is currently running that includes a combination of in vitro and in vivo assays in order to identify and classify substances in relation to their potential to interact with the endocrine systems (tier 1) and then to develop concentration response curves in animal models (tier two, under validation) [423]. The ultimate goal is to clarify the biological responses of these compounds in whole organism, but in order to test approximately 80,000 compounds, millions of animals should be sacrificed. In view of the ethical and economical problems, animals have been replaced by cell lines or simplified systems (i.e., yeast); compared to the complexity of an organism, often the conclusions drawn are different from in vivo experiments [424, 425].
Pretests of new chemicals before they are marketed and a group classification of the EDCs based on their chemical biochemical and biological activities should be done; the problem is difficult to resolve since there are no adequate tools to test complex mixtures.
References
1.
Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA (1996) Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the USEPA-sponsored workshop. Environ Health Perspect 104(Suppl 4):715–740PubMed
2.
Katzenellnbogen JA (1995) The structural pervasiveness of estrogenic activity. Environ Health Perspect 103(suppl 7):99–101
3.
Hammond GL (1995) Potential functions of plasma steroid-binding proteins. Trends Endocrinol Metab 6:298–304PubMed
4.
Apter D, Bolton NJ, Hammond GL, Vihko R (1984) Serum sex hormone-binding globulin during puberty in girls and in different types of adolescent menstrual cycles. Acta Endocrinol (Copenh) 107:413–419
5.
Belgorosky A, Rivarola MA (1986) Progressive decrease in serum sex hormone-binding globulin from infancy to late prepuberty in boys. J Clin Endocrinol Metab 63:510–512PubMed
6.
Hammond GL, Langley MS, Robinson PA, Nummi S, Lund L (1984) Serum steroid binding protein concentrations, distribution of progestogens, and bioavailability of testosterone during treatment with contraceptives containing desogestrel or levonorgestrel. Fertil Steril 42:44–51PubMed
7.
Colborn T (2004) Endocrine disruption overview: are males at risk? Adv Exp Med Biol 545:189–201PubMed
8.
Kinlay M, Plant JA, Bell JN, Voulvoulis N (2008) Endocrine disrupting pesticides: implications for risk assessment. Environ Int 34:168–183
9.
Golden RJ, Noller KL, Titus-Ernstoff L, Kaufman RH, Mittendorf R, Stillman R, Reese EA (1998) Environmental endocrine modulators and human health: an assessment of the biological evidence. Crit Rev Toxicol 28:109–227PubMed
10.
Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr, Jégou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE (1996) Male reproductive health and environmental xenoestrogens. Environ Health Perspect 104(Suppl 4):741–803PubMed
11.
Colborn T, Dumanoski D, Myers JP (1995) Our stolen future. Penguin, New York
12.
van Larebeke N, Hens L, Schepens P, Covaci A, Baeyens J, Everaert K, Bernheim JL, Vlietinck R, De Poorter G (2001) The Belgian PCB and dioxin incident of January–June 1999: exposure data and potential impact on health. Environ Health Perspect 109:265–273PubMed
13.
Carpenter DO, Arcaro KF, Bush B, Niemi WD, Pang S, Vakharia DD (1998) Human health and chemical mixtures: an overview. Environ Health Perspect 106(Suppl 6):1263–1270PubMed
14.
Damstra T, Barlow S, Bergman A, Kavlock R, Van der Kraak G (eds) (2002) International Programme on Chemical Safety: global assessment of the state-of-the-science on endocrine disruptors. WHO, Geneva. http://www.who.int/pcs/emerg_site/edc/global_edc_TOC.htm. Accessed 20 Oct 2013
15.
Welshons WV, Nagel SC, vom Saal FS (2006) Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147(6 Suppl):S56–S69PubMed
16.
Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N (1995) Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect 103:608–612PubMed
17.
Muncke J (2009) Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ 407:4549–4559PubMed
18.
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177PubMed
19.
Grund S, Higley E, Schönenberger R, Suter MJF, Giesy JP, Braunbeck T, Hecker M, Hollert H (2010) The endocrine disrupting potential of sediments from the Upper Danube River (Germany) as revealed by in vitro bioassays and chemical analysis. Environ Sci Pollut Res 18:446–460
20.
Rodgers-Gray TP, Jobling S, Kelly C, Morris S, Brighty G, Waldock MJ, Sumpter JP, Tyler CR (2001) Exposure of juvenile roach (Rutilus rutilus) to treated sewage effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ Sci Technol 35:462–470PubMed
21.
Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD, Servos M (1999) Behavior and occurrence of estrogens in municipal sewage treatment plants–I. Investigations in Germany, Canada and Brazil. Sci Total Environ 225:81–90PubMed
22.
Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, van Aerle R, Santos E, Brighty G (2006) Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect 114(Suppl 1):32–39PubMed
23.
Keiter S, Rastall A, Kosmehl T, Wurm K, Erdinger L, Braunbeck T, Hollert H (2006) Ecotoxicological assessment of sediment, suspended matter and water samples in the upper Danube river – a pilot study in search for the causes for the decline of fish catches. Environ Sci Pollut Res 13:308–319
24.
Gaido K, Dohme L, Wang F, Chen I, Blankvoort B, Ramamoorthy K, Safe S (1998) Comparative estrogenic activity of wine extracts and organochlorine pesticide residues in food. Environ Health Perspect 106(Suppl 6):1347–1351PubMed
25.
Borchers A, Teuber SS, Keen CL, Gershwin ME (2010) Food safety. Clin Rev Allergy Immunol 39:95–141PubMed
26.
Judd N, Griffith WC, Faustman EM (2004) Contribution of PCB exposure from fish consumption to total dioxin-like dietary exposure. Regul Toxicol Pharmacol 40:125–135PubMed
27.
Grimvall E, Rylander L, Nilsson-Ehle P, Nilsson U, Strömberg U, Hagmar L, Ostman C (1997) Monitoring of polychlorinated biphenyls in human blood plasma: methodological developments and influence of age, lactation, and fish consumption. Arch Environ Contam Toxicol 32:329–336PubMed
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


