http://evolve.elsevier.com/Haveles/pharmacology
Although drugs may act on biologic systems to accomplish desired effects, they lack absolute specificity in that they can act on many different organs or tissues. This lack of specificity is the reason for undesirable or adverse drug reactions. No drug is free from producing some adverse effects in a certain number of patients. It is estimated that between 5% and 10% of the patients hospitalized annually in the United States are admitted because of adverse reactions to drugs. Also, during their hospitalizations, 10% to 20% of patients experience adverse reactions to drugs.
The dental hygienist is in a good position to observe any adverse reactions to or undesirable effects of drugs administered in the dental office. Adverse reactions to drugs prescribed by the patient’s physician can be identified in the health history. The dental hygienist should question the patient about any potential oral manifestations of drugs. For example, if the patient is taking phenytoin (Dilantin), questions about enlargement of the patient’s gums should be explored. Because many drugs can produce xerostomia, complaints of dry mouth should direct the dental hygienist to examine the patient’s medications. Knowledge of the typical adverse drug reactions can help dental hygienist identify, minimize, or prevent these types of reactions. Because of the rapport between a patient and the dental hygienist, the patient often reveals important facts about the health history or asks questions concerning medications prescribed. The dental hygienist must know the terms used to describe adverse reactions to discuss a drug’s undesirable effect accurately with other health professionals. For example, allergy refers to a specific type of reaction to a drug but does not include a complaint of excessive gas, or flatulence.
Definitions and classifications
Unfortunately, every drug has more than one action. The clinically desirable actions are termed therapeutic effects, and the undesirable reactions are termed adverse effects. Dividing a drug’s effects into two categories is artificial because whether an effect is adverse or therapeutic depends on the indication for which the drug is being used. For example, when an antihistamine used to relieve hay fever causes drowsiness, the drowsiness can be considered an adverse effect. However, if the antihistamine were being used to induce sleep (over-the-counter [OTC] sleep aid), drowsiness would be considered the therapeutic effect.
An adverse drug reaction is a response to a drug that is not desired, is potentially harmful, and occurs at usual therapeutic doses. It may be an exaggeration of the desired response, an expected but undesired response, an allergic reaction, a cytotoxic reaction, or an effect on the fetus. Often, adverse drug reactions are divided into the following categories:
The importance of distinguishing between different types of adverse effects can be shown using aspirin as an example. Aspirin can cause adverse reactions such as gastric upset or pain. At higher doses, aspirin can predictably produce toxicity such as tinnitus and hyperthermia (elevated temperature). Another type of reaction to aspirin is allergic, often involving a rash or difficulty in breathing (asthma-like reaction). These differences are significant and become pertinent when one is discussing an adverse reaction with another health professional. Patients who experience allergic reactions to a medication should not receive that medication or similar medications. Side effects such as gastrointestinal upset, although bothersome, are not reasons to avoid prescribing a medication. It can be given. However, if the gastrointestinal upset is too much for the patient, another drug should be considered. It is important to describe in the patient’s chart the patient’s “problem” in enough detail so that side effects can be separated from allergic reactions. Figure 3-1 describes the types of adverse reactions and notes whether they are predictable or dose dependent.
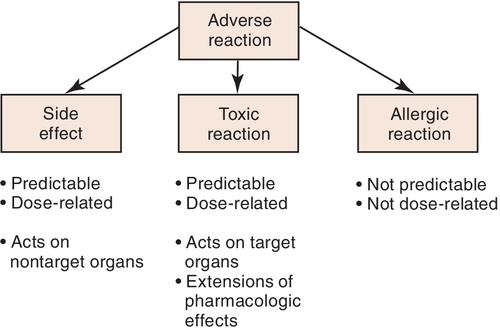
Clinical manifestations of adverse reactions
Before a drug is used, there must be an assessment of its risk against its benefits (risk-to-benefit ratio). This means that the beneficial effect of the drug must be weighed against its potential for adverse reactions. For example, one would compare the drug’s therapeutic effect (e.g., controlling seizures) with its potential to cause an adverse reaction (e.g., birth defects). In a real-life example, one should compare the therapeutic effect of certain drugs to produce weight loss with their potential for the serious adverse reactions such as primary pulmonary hypertension (which is fatal in 50% of patients) and cardiac valvular damage.
Exaggerated Effect on Target Tissues
An exaggerated effect on its target tissue or organ is considered an extension of the therapeutic effect caused by the overreaction of a sensitive patient or by the use of a dose that is too large. For example, a patient may experience exaggerated hypoglycemia when given a therapeutic dose of an oral hypoglycemic agent for the treatment of diabetes. The patient’s plasma glucose level may fall too low, because of an unusual sensitivity to the drug, the dose administered was too high for that patient, or the patient administered the drug but did not eat. Occasionally, this type of adverse reaction may result from liver or kidney disease. Because the disease interferes with the drug’s metabolism or excretion, the drug’s action may be enhanced or prolonged.
Effect on Nontarget Tissues
The effect on nontarget organs or tissues is caused by the nontherapeutic action of the drug. These reactions can occur at usual doses, but they appear more often at higher doses. For example, aspirin may produce gastric upset in usual therapeutic doses, but with higher doses, salicylism, characterized by tinnitus, disturbances in the acid-base balance, and confusion, can result. Toxic reactions can affect many parts of the body. A reduction in the dose of a drug usually reduces these adverse reactions.
Effect on Fetal Development (Teratogenic Effect)
The word teratogenic comes from the Greek prefix terato-, meaning “monster,” and the suffix -genic, meaning “producing,” or “producing a malformed fetus.” The relationship between drugs and congenital abnormalities has been recognized since the middle of the twentieth century. In 1961, thalidomide, an OTC drug marketed in Europe, was found to cause phocomelia (short arms and legs) in the exposed fetus. In some cases, only one dose of this drug had the effect. This incident reinforced the fact that more studies were needed to determine the effects of drugs on pregnant women. For new drugs, many more studies on animals and their reproductive capacity are conducted before the drugs are put on the market. Although more information is now available about the safety of drugs in pregnant women, sufficient information is still lacking.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


