Chapter 9
Sleep-Related Breathing Disorders
Snoring and Obstructive Sleep Apnea
Sleep-related breathing disorders constitute a spectrum of clinical entities with variations in sleep structure, respiration, and blood oxygen saturation. The spectrum ranges from mild, intermittent snoring to severe obstructive sleep apnea (OSA) (< ?xml:namespace prefix = "mbp" />
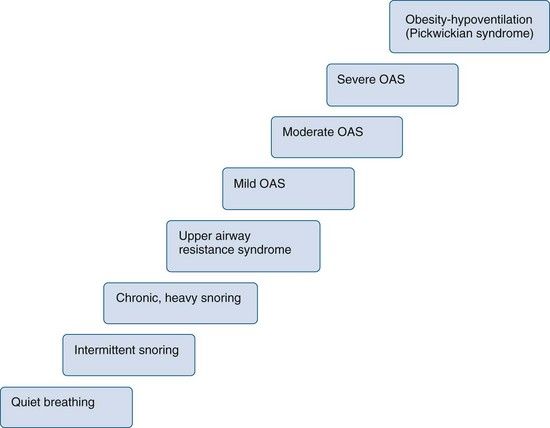
FIGURE 9-1 Clinical spectrum of sleep-related breathing disorders. OSAS, Obstructive sleep apnea syndrome.
(Redrawn from Phillips B, Naughton MT: Fast facts: obstructive sleep apnea, Oxford, 2004, Health Press Limited.)
Snoring, upper airway resistance syndrome (UARS), and OSA are the main subjects of this chapter. All of these sleep-related breathing disorders are due to upper airway obstruction of variable degree, leading to resistance to airflow during respiration. Attempts to breathe continue despite the obstruction. A related disorder, central sleep apnea, is the cessation of breathing that is due to disruption of central nervous system ventilatory drive; this type of apnea usually is associated with an underlying medical problem such as heart failure and is not due to obstruction, so it is not included in this chapter.
Snoring may occur alone or may be due to a more significant airway impairment. Snoring is the result of vibration of the soft tissues of the upper airway, primarily during inspiration. Primary snoring is sometimes referred to as simple snoring or benign snoring. It occurs as an independent entity and is not associated with disrupted sleep or complaints of daytime sleepiness. Findings on an overnight sleep study, or polysomnogram (PSG), are normal. Snoring occurs without abnormal ventilation. UARS is a clinical entity midway between primary snoring and OSA that is characterized by snoring, complaints of daytime sleepiness, and fragmentation of sleep. A PSG demonstrates some increase in ventilatory efforts, but the impairment is not severe enough to be classified as OSA. OSA is characterized by loud snoring and excessive daytime sleepiness with episodes of complete cessation of breathing (apnea) or significantly decreased ventilation (hypopnea) due to airway obstruction during sleep, along with significant fragmentation of sleep architecture. A PSG demonstrates abnormalities in sleep architecture, ventilation, and blood oxygen saturation.
General Description
Epidemiology
Snoring is extremely common in both genders and in all age groups. It is reported to occur in nearly 50% of the adult population, with a higher prevalence among men.
The reported prevalence of OSA varies widely because of differences in assessment methods and in the number of abnormal respiratory events per hour used to define abnormality. It is estimated that about 2% to 4% of the adult population 30 to 60 years of age is affected by OSA; however, 9% of women and 24% of men have signs or symptoms suggestive of sleep-disordered breathing.
Etiology and Pathophysiology
The underlying defect in sleep-related breathing disorders is an anatomically narrowed upper airway combined with pharyngeal dilator muscle collapsibility. The exact pathogenesis, however, is not well understood. Depending on the extent of narrowing, increased resistance to airflow may be clinically expressed as vibration of soft tissues (snoring), reduced ventilation (hypopnea), or complete obstruction (apnea).
Anatomic narrowing may occur at any site in the upper airway from the nasal cavity to the larynx. Within the nasal cavity, septal deviation and enlarged turbinates may cause narrowing. In the nasopharynx, hypertrophic adenoids and tonsils, an elongated soft palate, and an elongated and edematous uvula may be the cause. In the oropharynx, narrowing may be due to an enlarged tongue, retrognathia, excessive lymphoid tissue, palatine tonsils, or redundant parapharyngeal folds. The most common sites of airway narrowing or closure during sleep are the retropalatal and retroglossal regions.
In addition to anatomic narrowing of the airway, an abnormal degree of collapsibility is observed in the pharyngeal dilator muscles surrounding the airway. Patency of the airway depends on a balance between air pressure within the airway and pressure outside of the airway exerted by the parapharyngeal musculature. Muscles that surround the airway receive phasic activation during inspiration and tend to promote a patent pharyngeal lumen by dilating the airway and stiffening the airway walls.
Complications and Outcomes
To appreciate the consequences of sleep-related breathing disorders, it is necessary to review the aspects of normal sleep. Normal sleep patterns vary with age but are nevertheless similar across patient groups; thus, for illustrative purposes, the sleep of young adults is discussed here. Normal sleep occurs in two phases: non–rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep
TABLE 9-1 Percentage of Time Spent in the Various Stages of Sleep for Normal, Healthy Young Adults
| Stage Plus EEG Characteristics | Percent of Sleep |
|---|---|
| Relaxed wakefulness | <5% |
| Non–rapid eye movement sleep (NREM) | |
| Stage 1: transitional; easy arousal | 2-5% |
| Stage 2: sleep onset; K-complexes (sleep spindles) | 45-55% |
| Stage 3: high-voltage, high-amplitude slow waves | 3-8% |
| Stage 4: increased numbers of high-voltage slow waves | 10-15% |
| Rapid eye movement sleep (REM) | 20-25% |
| Associated with desynchronized brain waves on EEG, muscle atonia, bursts of rapid eye movement |
EEG, Electroencephalogram.
The phases of sleep are characterized by distinctive patterns on the electroencephalogram (EEG), as well as by the presence or absence of eye movements. NREM sleep occurs in three (or four) stages and generally is characterized by synchronous brain waves, mental inactivity, and physiologic stability (
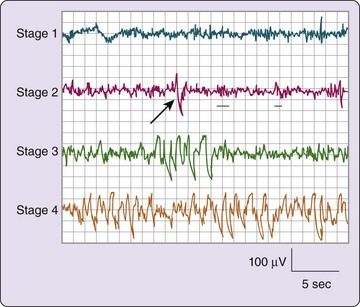
FIGURE 9-2 Electroencephalographic tracings of non–rapid eye movement (NREM) sleep stages.
(From Carskadon MA, Dement WC: Normal human sleep: an overview. In Kryger MH, Roth T, Dement WC, editors: Principles and practice of sleep medicine, ed 5, St. Louis, 2011, Saunders.)
After a period of NREM sleep, a “lightening” occurs, marked by entry into REM sleep. REM sleep is very different from NREM sleep and is characterized by asynchronous brain waves, an active brain, and physiologic instability, and muscular inactivity. The REM sleeep state often is described as “an active brain in a paralyzed body.” A key feature is the presence of periodic rapid movement of the eyes with low-voltage EEG waves resembling those typical of wakefulness (
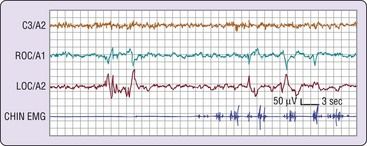
FIGURE 9-3 Phasic events in human rapid eye movement (REM) sleep. C3/A2 is an electrooculographic (EOG) lead. ROC/A1 is a lead from the outer canthus of the right eye, and LOC/A2 is another lead from the outer canthus of the left eye. Note the several bursts of activity in the eye lead tracings.
(From Carskadon MA, Dement WC: Normal human sleep: an overview. In Kryger MH, Roth T, Dement WC, editors: Principles and practice of sleep medicine, ed 5, St. Louis, 2011, Saunders.)
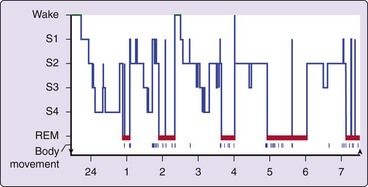
FIGURE 9-4 Histogram showing progression of sleep stages across a single night in a normal, healthy, young adult volunteer.
(From Carskadon MA, Dement WC: Normal human sleep: an overview. In Kryger MH, Roth T, Dement WC, editors: Principles and practice of sleep medicine, ed 5, St. Louis, 2011, Saunders.)
To gain the restorative benefits of sleep, it is necessary to cycle through the normal stages of sleep. NREM sleep provides physical restoration, whereas REM sleep provides psychic restoration. If such restoration does not occur because of sleep deprivation or sleep fragmentation, cognitive and physiologic disturbances will result. Within the spectrum of sleep-related breathing disorders, different outcomes may be seen. With primary snoring, the degree of airway resistance is such that vibration of the parapharyngeal soft tissues is the only result. No sleep fragmentation or disruption occurs, and no other impairment of airflow or oxygenation is noted. Generally accepted thought has been that primary snoring has no significant adverse health effects, but evidence now suggests that primary snoring may be a risk factor for type 2 diabetes, hypertension, carotid atherosclerosis, and stroke.
With OSA, increasing resistance to airflow occurs to the point of partial (hypopnea) obstruction or complete (apnea) obstruction and the cessation of breathing, despite efforts to continue to breathe. Depending on the degree and duration of the obstruction, hypoxia, anoxia, and hypercarbia may occur. These changes lead to CNS arousal and transition to a lighter stage of sleep (stage 1 or 2), stimulating partial awakening, relief of the obstruction, and resumption of breathing. Depending on the frequency of arousals during the night, sleep can be fragmented (
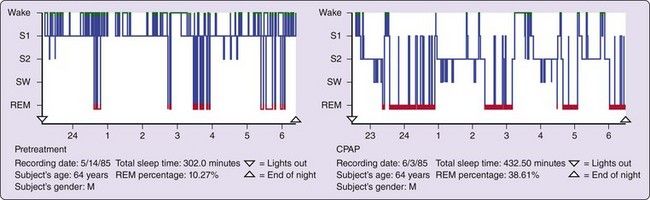
FIGURE 9-5 These sleep histograms show sleep study data for a 64-year-old male patient with obstructive sleep apnea syndrome. The left graph shows the sleep pattern before treatment. Note the absence of slow wave sleep (SWS), the preponderance of stage 1 (S1), and the very frequent disruptions. The right graph shows the sleep pattern in this patient during the second night of treatment with continuous positive airway pressure (CPAP). Note that sleep is much deeper (with more SWS) and more consolidated, and rapid eye movement (REM) sleep in particular is abnormally increased. The pretreatment REM percentage of sleep was only 10%, versus nearly 40% with treatment
(From Carskadon MA, Dement WC: Normal human sleep: an overview. In Kryger MH, Roth T, Dement WC, editors: Principles and practice of sleep medicine, ed 5, St. Louis, 2011, Saunders.)
Neurocognitive effects of OSA include sleepiness, decreased alertness, irritability, poor concentration, lack of libido, and memory loss. These deficits can lead to poor job performance, marital discord, interpersonal conflicts, and driving impairment. Up to 30% of traffic accidents involve sleepy drivers.
In addition to neurocognitive impairment, OSA is associated with numerous cardiovascular effects, including hypertension, stroke, congestive heart failure, pulmonary hypertension, and cardiac arrhythmia. OSA, which is now recognized as one of the treatable causes of hypertension,
Clinical Presentation
Signs and Symptoms
The signs and symptoms of sleep-related breathing disorders are those most often described by the bed partner or parent of a patient; they include snoring, snorting, gasping, and breath holding. Snoring is very common, as was previously indicated, and is the most common symptom in patients with OSA. However, most people who snore do not have OSA, but almost all patients with OSA snore. In the Wisconsin Sleep Cohort Study of subjects aged 30 to 60 years, 44% of men and 28% of women were habitual snorers, but only 4% of the men and 2% of the women had OSA.
Snoring may be very loud and disruptive to other members of the household. When snoring is the only complaint, the problem most often is primary snoring. If snoring is accompanied by daytime sleepiness and no breathing changes during sleep, UARS must be considered. Snoring accompanied by snorting, choking, gasping, or a complete cessation of breathing is likely to be a sign of OSA. Of note, however, definitive diagnosis of sleep-related breathing disorders cannot be made on the basis of clinical signs and symptoms alone.
Complaints of excessive daytime sleepiness are common in patients with OSA but are not specific, and the problem may be multifactorial. A commonly used subjective measure of sleepiness is the Epworth Sleepiness Scale

FIGURE 9-6 Epworth Sleepiness Scale.
(Redrawn from Johns MW: A new method for measuring daytime sleepiness: the Epworth sleepiness scale, Sleep 14:540-545, 1991.)
Obesity is common among patients with OSA. Obesity increases the risk of OSA several-fold; the most marked effects are observed during middle age.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


