chapter 9
General Considerations in Managing Orofacial Pains
The conditions that generate pain are susceptible to modification. The treatment of people who suffer pain entails the manipulation of those factors that initiate and accentuate pain and/or the institution of means and methods by which patients can better cope with their complaint. The conditions that we may set out to manipulate, therefore, comprise on the one hand such factors as the elimination of etiologic noxious stimuli, the interruption of nociceptive circuits, and the enhancement of the natural mechanisms of pain inhibition. On the other hand, we may elect to direct the main attention toward the patient rather than his or her problem in an effort to help the patient contend more successfully with the “disease.” Sternbach1 has shown that whereas “pain” in acute pain is a symptom of disease, “pain” in chronic pain is the disease itself. Instantly, it should be obvious that pain management is a complex undertaking. Unfortunately, we are just beginning to learn the rules of the game. This much we do know: it is the patient’s body that does the healing, not the doctor. The most that we can do is provide favorable conditions for healing. Our patients must understand that it is they, not we, who determine the final outcome of therapy. Healing is an activity that only the patients can do. We cannot do it for them.
The first step in the treatment of any condition is accurate and complete diagnostic evaluation of what the problem is, what structures are involved, and what conditions account for it. The diagnosis of pain is not a “naming process.” It is an understanding of pain genesis, modulating conditions, inhibitory and excitatory mechanisms, secondary effects, accompanying symptoms, and physical and emotional reactions that occur in human beings as distinct individuals. Every patient who presents a pain complaint has a problem that is peculiar to him or her. Yet we as dental practitioners are trained as therapists more than as diagnosticians. We are conditioned to treat, and our patients are conditioned to expect decisive and effective treatment from us. We are more mouth-oriented than patient-oriented. We do well as long as everything is obvious; when we face obscure problems, our conditioning may fail us. Therefore, when we meet an obscure pain complaint, we need to call forth all our best diagnostic capabilities.
It is essential at the very outset to decide whether we are dealing with an acute pain or a chronic pain. We must decide whether the behavioral characteristics suggest somatic or neuropathic pain, whether the pain emanates from superficial or deep structures, and whether the pain is primary or secondary. To skip these basic steps is to invite failure. Very early we need to decide whether the complaint appears to be stimulus-evoked. Will our therapy be directed toward the elimination of a source of noxious stimulation? Will it use techniques that manipulate modulating influences? Will it entail the management of neuropathic pain sources? Will it employ the use of systemic medications? Will psychotherapy likely be utilized? A decision is needed: “Is this a problem that I can handle alone, or do I need help? What kind of help, and from whom?” Ultimate success or failure may well rest upon such early decisions. They should form the overall guidelines for managing the problem at hand. Each case should be individualized. We are not dealing so much with different kinds of problems as with different kinds of people. Genuine caring on the part of the clinician is the one indispensable attribute in good pain management.2
Cause-Related Therapy
Many patients who experience orofacial pain have an organic structural cause that is responsible. The main thrust of therapy for such complaints is to identify the cause and eliminate it. The extent to which one achieves this will determine his or her success in the management of the pain problem. The cause of pain emanating from somatic structures is thought to be due chiefly to the action of algogenic substances, such as bradykinin, histamine, serotonin (5-hydroxytriptamine [5HT]), potassium, and phosphates.3 The etiology of neuropathic pain is less well understood.
Somatic Pain
Much somatic pain results from trauma, intrinsic injury, infections, and diseases that induce inflammatory reactions. The variety of traumatic conditions, with respect to the type of agent as well as the extent and location of injury, is nearly endless. Inflammatory conditions reactive to abusive use; intrinsic strains; microtraumas; bacterial, fungal, or viral infections; pathologic lesions; and biomechanical dysfunctions are almost without number. At a dental level, inflammatory conditions of the teeth, the supporting structures, and the mucogingival tissues as well as dysfunctional and inflammatory conditions of the temporomandibular joints (TMJs) and masticatory musculature are commonplace. It is known that ischemia liberates bradykinin,4,5,6 that hemolyzed red blood cells in muscle tissue liberate potassium ions, and that phosphates, histamine, and 5HT are liberated from blood platelets.7,8,9 Some of the visceral somatic structures elicit pain without such evident cause. Vascular pain involves more than simple vasodilation; it is likely that algogenic substances are involved.10,11,12
There are occasions when the somatic condition that generates pain is refractory to therapy, and the complaint persists as chronic structural pain. Cancer presents this problem. Some masticatory pains, such as inflammatory arthritis, may also fall into this category.
Therapeutic Modalities
Table 9-1 lists the many different approaches that should be considered when managing orofacial pain disorders. The appropriateness of each modality is determined by the specific diagnosis. Each modality will be reviewed in this chapter. The specific use of these modalities will be discussed in the chapters that review each orofacial pain disorder.
Pharmacologic Therapy
Although analgesics are the drugs used most frequently in the management of patients in pain, several other classes of pharmaceuticals are useful for palliative and cause-related therapy. Some drugs bind to known receptors, which are cellular components with which the natural body chemicals (ligands) interact to produce physiologic responses. Some drugs (agonists) mimic rather closely the action of the natural substances. Other drugs (antagonists or blocking agents) prevent those actions. For example, morphine (agonist) binds to certain receptors (mu and kappa) in the central nervous system (CNS) that normally interact with the natural endorphins (ligands); this interaction is prevented by naloxone (antagonist or blocking agent).
Some drugs act by neutralizing the effect of certain enzymes, thus producing a physiologic effect indirectly. The class of antidepressant drugs known as monoamine oxidase (MAO) inhibitors act to increase the concentration of 5HT, dopamine, and norepinephrine in the cerebrospinal fluid (CSF) by reducing their natural breakdown through an inhibitory action on the enzyme MAO. Aspirin produces an anti-inflammatory effect by inhibiting the action of the enzyme cyclooxygenase (COX), which normally metabolizes arachidonic acid into prostaglandins as the result of local tissue injury. Other drugs act in still different ways, and the action of many is not known.
Since drugs induce both intentional (primary) and unintentional (secondary) effects, the selection of the best drug in a given situation requires careful consideration of the various effects that may occur. When two or more drugs are ingested, their possible interactions should be understood. Some combinations are clearly incompatible; some drugs potentiate the action of others; some act synergistically. To properly gauge dosage, one should be familiar with the estimated half-life of the medication being used, especially if a sustained effect is desired. A drug’s half-life is the time necessary for the plasma concentration to decrease by 50%. A relatively steady blood level can usually be achieved after five doses are administered at periods that correspond to the drug’s half-life.13
Table 9-1 Therapeutic Modalities for the Management of Orofacial Pain Disorders
Medications of different kinds play an important role in the management of patients with pain. Their proper use is a major concern. It is the clinician’s responsibility to be adequately familiar with the drug being administered and with the patient who is receiving it, so that safety and effectiveness are ensured. Individualization is necessary.
Many things need to be known about a medication: indications and contraindications for its use, drug incompatibilities, mode of administration, mode of breakdown or metabolism, safe and toxic dosages, side effects, and possible complications. The clinician should be prepared to recognize and effectively deal with such things as sensitivity to the drug, undesirable side effects, toxicity, idiosyncrasy, anaphylaxis, and other untoward reactions to its use. He or she should know about the possibility of potentiation of other medications being used, synergisms, dependence, and the probability of addiction.
Adequate medical supervision and emergency care should always be available if needed. All these responsibilities rest firmly upon the prescribing doctor, and they cannot be abrogated. If the clinician is not prepared to accept these responsibilities, he or she should place pharmacologic management of the patient in the hands of someone who is.
Analgesic agents
Medications that reduce pain are an important class of drugs useful in pain control. As a general rule, the objective of analgesics should not be to eliminate pain altogether; instead, they should make the pain tolerable, since pain has some value in monitoring progress in the patient’s condition. It helps guide the patient as to when his or her actions are excessive or abusive. The American Pain Society13 has classified analgesic drugs into three groups: nonnarcotic analgesics, narcotic analgesics, and adjuvant analgesics.
Nonnarcotic analgesics
The nonnarcotic analgesics, which include aspirin and the nonsteroidal anti-inflammatory drugs (NSAIDs), have four major actions: analgesic, antipyretic, antiplatelet, and anti-inflammatory. They differ from narcotic analgesics in three ways: (1) they presumably prevent the formation of prostaglandin E by inhibitory action on the enzyme COX; (2) they do not produce tolerance, physical dependence, or addiction; and (3) they have a ceiling effect, whereby increasing the dosage beyond peak limits does not increase the analgesic effect, although it may affect its duration. Representative of such analgesics are aspirin, ibuprofen (Motrin), and ketoprofen (Orudis), which have relatively short half-lives, and nabumetone (Relafen) and naproxen (Naprosyn), which have relatively longer half-lives and therefore are longer-acting.
Also included in the nonnarcotic group are acetaminophen, which has no antiplatelet or anti-inflammatory action, and choline magnesium trisalicylate, which lacks antiplatelet action.13 The nonnarcotic analgesics are general-purpose medications indicated for use in the treatment of mild to moderate pain and chronic cancer pain. Aspirin and the NSAIDs prolong bleeding time and therefore are contraindicated with anticoagulant therapy and other coagulation deficiency conditions. It is thought that the antiplatelet action is beneficial to those at risk for cardiac attacks. Gastric irritation is a common side effect of these drugs. Acetaminophen is approximately equipotent to aspirin but has no antiplatelet or anti-inflammatory action.
Prostaglandin E2 is metabolized from arachidonic acid through action of the enzyme COX. There are two identified isomers of COX: COX1 and COX2. The action of COX1 produces prostaglandins that play a physiologic “housekeeping” role in renal parenchyma, gastric mucosa, platelets, and other tissues. It aids in maintaining normal function. Inhibition of COX1 is responsible for gastrointestinal toxicity. COX2 is present in most tissues in small amounts. It is expressed primarily in sites of inflammation and produces prostaglandins that are involved in inflammation and mitogenesis. Therefore, if COX2 is inhibited, the inflammatory response is reduced, which results in less nociception and pain.14,15,16 In recent years there has been a strong interest in the “COX2 inhibitor” drugs, which may reduce pain without the adverse side effects of gastrointestinal irritation. An interesting finding is that COX2 may also play a role in pain inhibition in the CNS.17,18 Examples of COX2 inhibitor drugs available are celecoxib (Celebrex), rofecoxib (Vioxx), and valdecoxib (Bextra).
Narcotic analgesics
Morphine-like drugs act through CNS receptors to induce a peripheral analgesic effect. 19,20 They depress nociceptive neurons while stimulating nonnociceptive cells.21 Both aspirin and morphine inhibit the release of bradykinin, but aspirin inhibits its release regardless of the type of noxious stimulus, while morphine inhibits its release when mediated by neural mechanisms. 22 There is evidence that part of morphine-induced analgesia is contributed indirectly by the release of endogenous opioid peptides.23 There is also some evidence that opiates exert an inhibitory influence on the release of substance P.24 There is no hard evidence that the addition of codeine to NSAIDs yields any measurable increase in analgesic effect.25 As with all drug therapy, it is the responsibility of the prescribing practitioner to know the proper dosage, indications, contraindications, incompatibilities, and signs of toxicity and/or side effects of prescribed medications. The prescribing clinician should be prepared to cope with any ill effects induced by narcotic therapy.
Narcotic analgesics comprise three classes of drugs: morphine-like agonists, mixed agonists-antagonists, and partial agonists. Included among the morphine-like agonists are codeine, hydrocodone, oxycodone, meperidine, propoxyphene, morphine, hydromorphone, methadone, levorphanol, oxymorphone, and heroin. Representative of the mixed agonist-antagonist drugs are pentazocine, nalbuphine, and butorphanol. Buprenorphine is a partial agonist.
Narcotic medications cause constipation, and therefore stool softeners and laxatives may need to be provided. The clinician should carefully individualize the selection of drug, the mode of administration, and the dosage. Narcotics are best administered on a regular time schedule, rather than as needed, to minimize unnecessary pain periods that may require increased dosage attendant with overmedication and increased toxicity. Side effects should be recognized and treated appropriately. The clinician should be watchful for tolerance, physical dependence, and/or addiction (psychologic dependence). Tolerance means that larger doses are required to obtain a satisfactory analgesic effect. Physical dependence means that withdrawal symptoms accompany abstinence. Addiction means compulsive craving for the drug and the need to use it for effects other than the relief of pain.
Narcotics are useful in managing severe acute pain and chronic cancer pain but are not generally indicated for the management of most chronic orofacial pain disorders. However, there are a few orofacial pain conditions that are so poorly controlled that narcotic medications may be considered. Typically these conditions are continuous neuropathic pain disorders, where we now better understand the pain mechanisms but often still lack the ability to eliminate the pain. When narcotics are considered for long-term management of benign pain conditions, both the patient and the therapist need to be aware of all the risks and complications. Long-term narcotic use should not be taken lightly. Narcotics should be considered only when other medications, both analgesic and nonanalgesic, have failed to control the pain, and the quality of the patient’s life is so diminished that the therapist must step in and provide relief. When this occurs, a contract should be established between the patient and the therapist, clarifying the responsibilities of each. The therapist needs to commit to evaluating the patient on a regular basis (eg, monthly) to determine the appropriateness of the pain relief and evaluate any adverse side effects as well as the psychologic state of the patient. Depression is a common occurrence in chronic pain patients, and this condition needs to be regularly evaluated, and when indicated, managed. The patient needs to agree to take the medication only as directed and not change any dosage without the consent of the therapists. The patient must agree to receive pain medication from the therapist only and keep the therapist informed of any change in his or her medical condition. The patient must agree in advance that he or she will submit to a drug screening test at any time the therapist feels it is in order. Any lost medication will not be replaced unless a full explanation is accepted by the therapist. Having the patient sign a formal narcotic contract before beginning long-term narcotic management helps emphasize to the patient the seriousness of the agreement. Any violation of the contract results in discontinuation of the use of narcotic medications. If the patient has been taking the medication for a long time, a treatment-withdrawal program is developed or the patient is sent to a formal drug rehabilitation center, whichever is deemed more appropriate.
Adjuvant analgesics
The drugs listed as adjuvant analgesics are those that enhance the analgesic effect of other medications or have independent analgesic activity in certain situations. They are not primarily used as an analgesic, but in certain conditions with certain disorders they may reduce the pain experience. These include tricyclic antidepressants,26 antihistamines, caffeine, dextroamphetamine, steroids, phenothiazines, and anticonvulsants. Many of these will be discussed in the following pages.
Anesthetic agents
Local anesthetics have an essential role in pain management, both diagnostically and therapeutically. Anesthetic agents can be used either topically or within the tissues by injection.
Topical anesthetics
Topical anesthetics may be used as solutions, sprays, ointments, or lozenges. Water-soluble ointments containing a topical anesthetic and a germicide are useful for managing dental alveolitis.
Analgesic balms are agents that give soothing, palliative relief of inflammatory pain of both superficial and deep somatic categories when applied topically to exposed or ulcerated tissue. Aloe vera juice is an ancient remedy for superficially generated inflammatory pain. It is an ingredient of compound benzoin tincture. Balsam of Peru, eugenol, and guaiacol are other well-known balms. Applied in various forms as liquid, ointment, or cement-like or adhesive dressings, analgesic balms are extremely useful in the palliative control of pain emanating from exposed or ulcerated cutaneous and mucogingival tissues, exposed dentin, and acute alveolitis.
Sometimes topical anesthetics such as lidocaine or benzocaine can be mixed with other medications to enhance the pain-relieving effect. For example, in some peripheral neuropathies a combination of lidocaine, amitriptyline, and carbamazepine (Tegretol) can provide pain relief. When this is found to be helpful and the painful area is intraoral, a dental stent can be fabricated to place over the painful area so that the medication will be maintained in the correct area for a longer period of time (Figs 9-1a and 9-1b).
Injectable local anesthetics
A variety of injectable local anesthetics are available in different concentrations, both with and without a vasoconstricting agent. Long-acting local anesthetics such as bupivacaine hydrochloride (Marcaine) are useful, although they entail a higher risk of toxicity. Proper dosage, correct technique, adequate precautions, and readiness for emergencies are essential to the safety and effectiveness of all local anesthetics. Resuscitative equipment, oxygen, and other resuscitative drugs should be instantly available, and adequate training in their proper use is necessary. Since most untoward reactions with local anesthetics follow accidental intravascular injection, aspiration prior to injection of any medication is a standard must.
The use of local anesthesia to control surgical pain and diagnostically to identify nociceptive pathways and primary sources of pain is commonplace (Fig 9-2). The therapeutic use of analgesic blocking is less well known. Four facets of this modality of pain control are useful at a clinical level, namely: (1) to arrest primary pain input, (2) to interrupt pain cycling, (3) to resolve myofascial trigger point activity, and (4) to induce a sympathetic blockade.
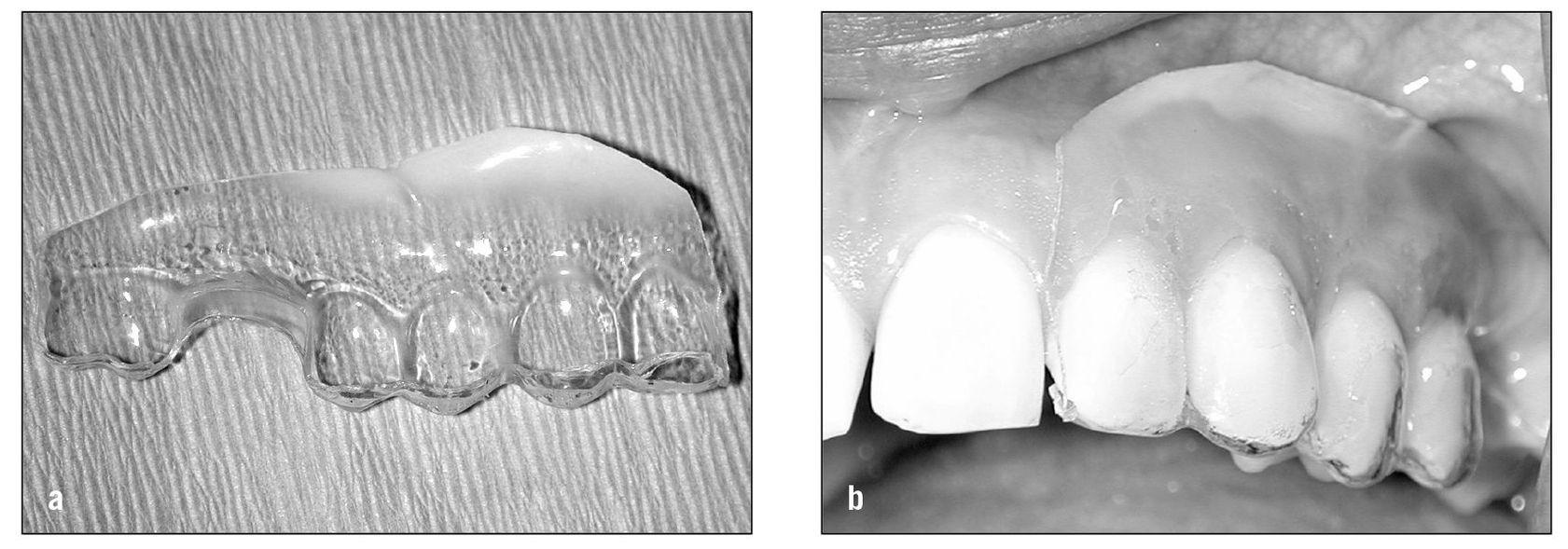
Fig 9-1 (a) When topical medications are used in the mouth, a stent can be fabricated that can be used to shield the medication from the tongue and saliva. (b) The medication is placed onto the involved tissue and the stent is placed over the teeth to keep the medications in place longer.
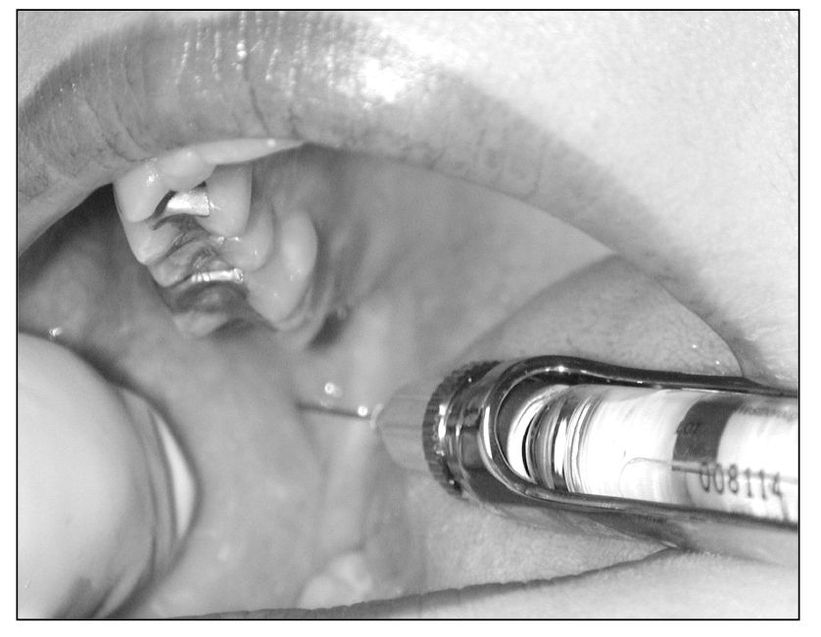
Fig 9-2 An inferior alveolar nerve block can be a very important tool to differentiate primary pain from heterotopic pain. It is especially helpful in identifying toothache, since this block does not anesthetize other common sources of pain such as the muscles or joints.
Arresting primary pain input. Primary deep pain input is an important factor, not only in initiating heterotopic pains and secondary muscle effects, but also in perpetuating them. For example, the pain of local muscle soreness by its own central excitation tends to perpetuate cyclic muscle pain (see chapter 12). The arrest of cyclic muscle pain by analgesic blocking has marked therapeutic value; it shuts off this perpetuating influence. This can be done by anesthetizing the muscle itself (Fig 9-3). Since myotoxicity of local anesthetics may restrict the choice of agent to short-acting anesthetics of low potency, it is preferable to block the nociceptive pathway rather than the muscle proper. Long-acting, potent anesthetics can then be used with relative impunity, and thus the benefits of arresting the deep pain input can be extended.
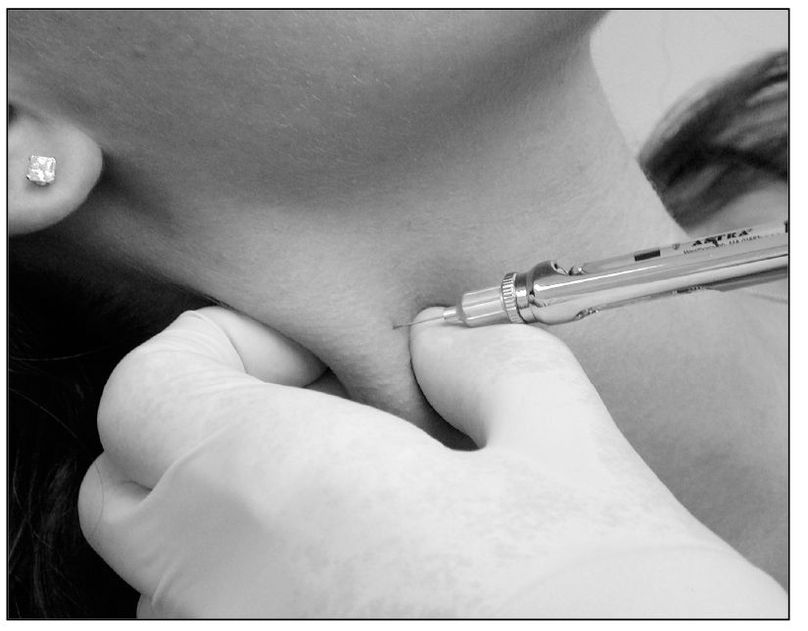
Fig 9-3 An injection of local anesthetic into the sternocleidomastoid muscle can be useful to help differentiate primary pain from heterotopic pain. When heterotopic pain is present, this injection will reduce the pain even in a remote site, such as the TMJ.
Interruption of pain cycling. On the basis of clinical evidence, a cycling mechanism appears to complicate several pain disorders that occur in the orofacial region. When such cycling is effectively interrupted by local anesthetic blockage of nociceptive impulses at the primary source or somewhere along its mediating pathway, remission of pain may significantly outlast the period of anesthesia. It is thought that this prolonged relief may be the result of a decrease in the sensitization of the second-order neuron after the nociceptive input is terminated. Such remission indicates that a therapeutic effect has been achieved. When such an effect can be obtained with a short-acting anesthetic, a greater therapeutic effect usually results from the use of a longer-acting drug.
Repeated analgesic blocking of the pathway that subserves the peripheral receptors that trigger trigeminal neuralgia may induce an extended period of remission. Analgesic blocking of the primary source of pain input may effectively arrest some myofascial trigger point pains in the facial region. Myospasm of masticatory muscles can be influenced favorably, if not resolved completely, by one or more analgesic blocks of the nociceptive pathway that serves the muscle.

Fig 9-4 When myofascial pain is present, a trigger point in the trapezius muscle can produce pain referral in the ipsilateral face and temple area. An injection of local anesthetic in the trapezius trigger point can reduce the pain in this area as well as reduce the heterotopic face pain.
Trigger point therapy. It is well known that the injection of a short-acting, aqueous local anesthetic of low myotoxicity at a myofascial trigger point located in muscle tissue effectively resolves the referred pain phenomena that characterize myofascial pain syndromes27,28 (Fig 9-4). Several authors 29,30,31 believe that such therapy is required when the trigger point is located in a muscle tendon. The technique used for trigger point injections has been described in chapter 8.
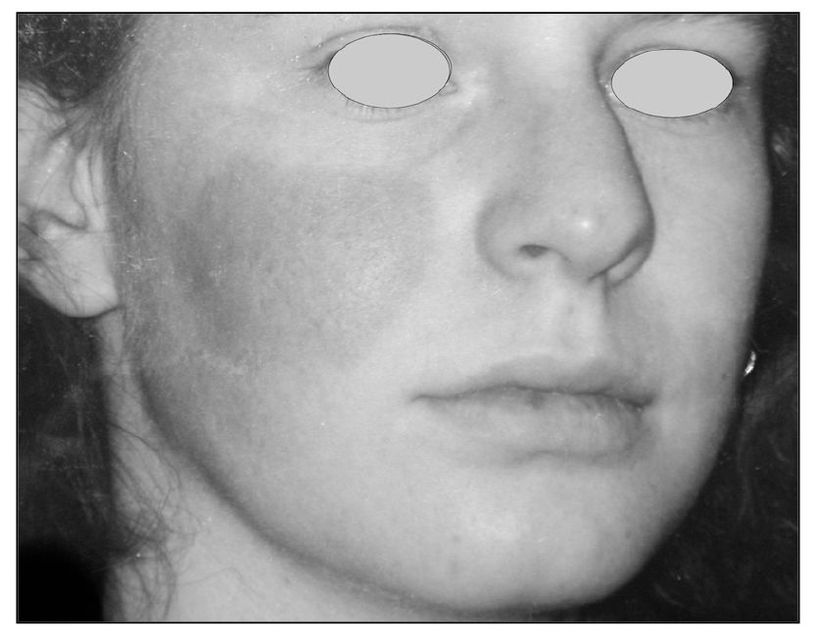
Fig 9-5 This patient reveals a very specific area of the check that is red and warm. This area has been affected by unusual sympathetic activity. When this is present, the clinician needs to evaluate the contribution of the sympathetic nervous system in the pain condition. A stellate ganglion block may be helpful to determine the role of the sympathetic nervous system in the pain condition (sympathetically maintained pain).
Sympathetic blockade. The diagnostic analgesic blocking of afferent sympathetic pathways to identify pain mediated by such neurons is well known (Fig 9-5). The stellate ganglion block is the one usually used for afferent sympathetic routes from the head.32,33,34,35 Analgesic blocking at the same site also arrests the efferent sympathetic impulses and constitutes an ipsilateral sympathetic blockade as far as the orofacial region is concerned. It is reported that daily stellate ganglion analgesic blocking is as effective as guanethidine for the treatment of reflex sympathetic dystrophy.33,36 Thompson 37 reported that half of his 120 cases of causalgia were relieved by repeated sympathetic analgesic blocks. Procacci et al38 reported that, in 11 of their 30 subjects with reflex sympathetic dystrophy of the limbs, a significant increase in cutaneous pain threshold developed after analgesic blocking of the sympathetic ganglia on the affected side. Wetchler and Wyman39 reported that all 23 of their patients with acute herpes zoster of the ophthalmic nerve had excellent pain relief and freedom from cutaneous scarring by treatment with daily analgesic blocks of the stellate ganglion. Three of this group, however, complained of postherpetic neuralgia at the 6-month follow-up.
The three pain disorders for which analgesic blocking of the stellate ganglion appears to be specifically indicated are (1) sympathetically maintained deafferentation pains, such as reflex sympathetic dystrophy 40,41,42,43,44; (2) herpes zoster45,46,47; and (3) postherpetic neuralgia.48 With both reflex sympathetic dystrophy and acute herpes zoster, early treatment appears to be the key to success. For postherpetic neuralgia, Milligan and Nash48 reported that 75% of their patients were helped if sympathetic blockade was performed within the first year.
Anti-inflammatory agents
In addition to the anti-inflammatory analgesics, several similar nonsteroidal medications are used chiefly for their anti-inflammatory effect. They are mildly analgesic and antipyretic. The specific action of all such medications is not known. The therapeutic effect appears to relate to the inhibition of prostaglandin biosynthesis. All such drugs should be used with caution, especially when protracted therapy is needed. Good medical supervision is appropriate. These agents do not alter the course of disease; they only suppress the symptoms of inflammation—hence their usefulness in the management of inflammatory pain.
Corticosteroids exert a potent anti-inflammatory effect, presumably by acting to inhibit prostaglandin biosynthesis. However, they also cause profound and varied metabolic effects and modify the body’s immune responses to diverse stimuli. They are eminently useful medications but require considerable care in administration. Extended use should be done only under close medical supervision. Their suppressive effect on inflammation may mask infection. They are contraindicated for systemic fungal and herpes simplex infections. Corticosteroid ointments are available in different strengths and with different bases. They are useful for topical application of the drug.
Devor et al49,50 reported that locally applied corticosteroid rapidly and effectively arrested the pain from damaged nerve fibers in experimental neuromas. They suggested that it acts directly on the membrane rather than as an anti-inflammatory.
Antidepressants
Some patients with neurovascular pain and patients with chronic pain who verbalize reactive depression may respond to the judicious use of antidepressant medications. The tricyclic antidepressants increase the availability of 5HT and norepinephrine in the CSF. The demethylated tricyclics make 5HT proportionately more available and induce some sedative effect. They are useful in treating agitated depression. The mono-methylated tricyclics make norepinephrine proportionately more available and induce some CNS stimulation. They are used more for retardant depression.51
Tricyclic antidepressants have been used with some success for a variety of chronic pain conditions.52,53,54,55 It has been demonstrated that a low dose of amitriptyline (10 mg) just before sleep can have an analgesic effect on chronic pain after several weeks of use.56,57,58,59 This clinical effect is not related to any antidepressive action, since antidepressive dosages are from 10 to 20 times higher. Although tricyclic antidepressants increase the available 5HT in the CSF, they may not have inherent analgesic properties. In normal subjects, they have no greater effect on pain than does placebo.60 Ward et al61 reported that all moderately to severely depressed individuals in this study complained also of pain, with headache being the most frequent complaint. Patients who obtained minimal antidepressant effect from tricyclics also obtained minimal analgesic effect.
The MAO inhibitors increase the available 5HT, norepinephrine, and dopamine in the CSF by inhibiting their breakdown. They potentiate the action of sympathomimetic substances and may induce a hypertensive crisis. The use of all antidepressant agents should be under adequate medical supervision.
Serotoninergic antidepressant medications are useful in the management of some chronic pain disorders.62,63,64 Antidepressant therapy, however, is not effective for deafferentation dysesthesia.65 Amitriptyline has a pain-relieving action in postherpetic neuralgia that is not primarily linked with its serotoninergic and antidepressant effect. 64,66 Although amitriptyline has a beneficial effect on some chronic pains, it does not appear to have the same positive effect on acute pain.67 It has been reported that subanalgesic doses of antidepressant drugs potentiate the action of morphine.68,69
Although the tricyclics have been helpful for some neuropathic pains, their side effect profile can be formidable. Common side effects are drowsiness and anticholinergic effects (ie, dry mouth, constipation). In the 1980s a new class of antidepressants was introduced: the selective serotonin reuptake inhibitors (SSRIs). These new antidepressants have better efficacy on mood disorders, with significantly fewer side effects. Some of the commonly used SSRIs are fluoxetine (Prozac), venlafaxine (Effexor), paroxetine (Paxil), sertraline (Zoloft), nefazodone (Serzone), fluvoxamine (Luvox), citalopram (Celexa), and escitalopram (Lexapro). As we study these drugs more completely, we appreciate that noradrenaline can also be affected and therefore subcategories are arising. For example, venlafaxine is a serotonin and noradrenergic reuptake inhibitor (SNaRI). Mirtazapine (Remeron) is considered a noradrenergic and specific serotoninergic antidepressant (NaSSA). Reboxetine (Edronax), a newer medication available in Europe, has its primary effect on the reuptake of noradrenaline and therefore is considered a noradrenaline reuptake inhibitor (NaRI). Venlafaxine, an SNaRI, has received the most investigation and has been shown to be effective in the treatment of different kinds of pain, with a side-effect profile that is significantly better than that of the tricyclic antidepressants.70 The other new antidepressants have been studied less extensively; thus, only anecdotal therapeutic results and experimental works have been found and reported. Further evidence-based research in the safety and efficacy of these promising agents for pain relief is warranted.
Caution should be exercised in the administration of tricyclic antidepressants, especially in patients with a history of cardiac disease. A potentially serious outcome may accompany overdosing as well as combinations with other drugs such as antihistamines, beta-blockers, calcium-channel blockers, SSRIs, and anticonvulsants.71 It is well that such medications be administered with medical supervision and consent.
Antianxiety agents
Numerous sedative and tranquilizing medications are available, some of which also have muscle relaxant action. The major tranquilizers, such as the phenothiazines, are very useful in pain control by reducing the modulating effects of anxiety and apprehension. They are not addictive. They may display some objectionable side effects, however, and therefore require careful monitoring. Extrapyramidal effects are not uncommon. Protracted use has caused tardive dyskinesia.
The minor tranquilizers, such as meprobamate and diazepam, have the advantage of fewer side effects. Their muscle relaxant action is also useful. But they present a serious potential for drug tolerance and for dependence, and abuse of such drugs is commonplace. When utilized for pain control, they are best prescribed for limited periods only. When protracted use is needed, different drugs should be used periodically so that neither dependence nor tolerance is permitted to occur. Tranquilizers appear to exert some potentiating effect on narcotic analgesics.72
Clonazepam is a benzodiazepine that may have some pain-reducing effects in certain neuropathic pain disorders.73,74 This medication has specifically been use with some success in burning mouth disorders.75,76
Vasoactive agents
Neurovascular pain disorders may be favorably influenced by the alpha-adrenergic blocking action of ergotamine tartrate, which causes a stimulating effect on the smooth muscle of peripheral and cranial blood vessels. It is available in several different preparations, both with and without the addition of caffeine. The caffeine enhances this vasoconstricting effect without increasing the dosage. Its effect appears to be greatest on the pulsatile component of neurovascular pain. The drug has several contraindications, including peripheral vascular and coronary heart disease, hypertension, and pregnancy.
Beta-adrenergic blocking agents that decrease vasodilator responses to beta-adrenergic receptor stimulants may have value in neurovascular pain disorders, even though the mechanism for the antimigraine effect has not been established.77 The successful use of beta-blockers in the treatment of phantom limb pain has also been reported. 78 Somatostatin has been reported to inhibit the effect of the release of substance P from peripheral sensory nerve terminals. Since substance P induces effects that resemble some of the symptoms of neurovascular pain disorders, such as cluster headache, the use of somatostatin has been investigated as a possible treatment modality. It is reported to be as effective as ergotamine tartrate in the treatment of cluster headache.79
5HT receptors have been strongly implicated in the mechanism of migraine headache. Many of the drugs that are effective in migraine are believed to affect one or more of the various 5HT receptors. Methysergide, cyproheptadine, and dihydroergotamine (DHE) interact with the 5HT2 receptor. Sumatriptan and DHE interact at the 5HT1A and 5HT1D receptors.80,81
Norepinephrine blockers
In lieu of analgesic blocking of the stellate ganglion for the control of sympathetically maintained pains about the orofacial region, the norepinephrine blocking agent guanethidine has been used.82 It appears to block the uptake of norepinephrine by sensitized deafferentated axons. The clinical benefit outlasts the pharmacologic actions. 83,84 Bonelli et al36 reported that IV administration of guanethidine once every 4 days for a total of four blocks was equal in effectiveness to daily stellate ganglion analgesic blocks for 8 days. In a controlled, randomized, double-blind, crossover study of 12 reflex sympathetic dystrophy patients, Lief et al85reported that weekly IV injections of guanethidine or reserpine achieved equally significant short-term pain relief. Levine and Basbaum86 reported that both guanethidine- and reserpine-induced sympathectomy decreased the severity of joint inflammation and pain, especially in rheumatoid arthritis.
Antimicrobial agents
A variety of antibiotic and other antimicrobial drugs is available for both topical and systemic administration. The choice depends on the type of organism represented in the infection. Culturing and susceptibility testing to identify the best-suited drug are good practice. The antinociceptive benefit of such agents relates to the resolution of the infection that causes pain.
It is very interesting to note that some antibiotics may actually have analgesic qualities.87,88 This can be very confusing to the patient and the clinician. For example, when a patient is treated with an antibiotic for a toothache and the pain resolves, it is immediately assumed that the antibiotic has eliminated the organism responsible for the infection and ultimately the pain. Although this is logical, it may not be true. The fact that an antibiotic reduces pain does not mean there is an infection present. Too often a patient is repeatedly placed on an antibiotic because it reduces the pain, and the clinician assumes the bacterial infection is produced by a resistant strain of organisms. Long-term antibiotic therapy creates significant risk factors for the patient, and such therapy should not be continued when pain relief is the only therapeutic effect. The clinician needs good evidence of an actual infection if antibiotic therapy is to be continued.
Antiviral agents
Acyclovir and famciclovir are effective drugs for treating infections caused by such viruses as herpes simplex (HSV-1, HSV-2) and herpes zoster.89,90,91,92,93 They may be administered topically,94 orally, or by IV. They appear to be more effective in the treatment of primary infections than recurrent episodes.95 Johnson reported that an experimental herpes vaccine proved effective in animal studies for both HSV-1 and HSV-2.96 Famciclovir (Famvir) is an orally administered prodrug of the antiviral agent penciclovir.93 Its unique pharmacokinetic profile makes it an efficacious, convenient, and well-tolerated alternative to the more traditionally prescribed acyclovir. Famciclovir is used for the acute treatment and suppressive therapy of recurrent genital herpes as well as for herpes zoster and its debilitating comorbidities. Famciclovir allows patients to manage or prevent symptoms, thereby significantly improving their quality of life. Its favorable safety profile makes it a good treatment choice for the elderly as well as for immunocompromised patients, including those infected with human immunodeficiency virus.
Antihistamine agents
Antihistamines counteract the vasodilator action of histamine by blocking certain histamine receptors. Many antihistamine preparations are available. They may be useful in allergic responses and some neurovascular pain disorders. A direct analgesic effect has been reported for several antihistamines (diphenhydramine, hydroxyzine, orphenadrine, pyrilamine).97,98,99,100,101 Combination of an antihistamine with acetaminophen has a greater analgesic effect than acetaminophen alone.101
Anticonvulsive agents
It is generally considered that anticonvulsive medications are useful in certain pain conditions, specifically neuropathic pains. An extensive review of the literature, however, fails to significantly support this assumption. 102 Nevertheless, the clinical judgment of many clinicians is that this class of medications helps neuropathic pain patients.
One of the oldest and still commonly used anticonvulsives is carbamazepine (Tegretol). Carbamazepine has been the mainstay medication for trigeminal neuralgia for many years. In fact, some neurologists suggest that if the pain associated with trigeminal neuralgia is not controlled with carbamazepine, then it is not trigeminal neuralgia. This is not a documented statement. Unfortunately, carbamazepine is associated with considerable side effects related to bone marrow suppression and blood dyscrasia. Pretreatment blood studies are necessary, and careful monitoring at regular intervals during treatment is essential for patient safety. Active medical supervision is a prerequisite during its use. There are a number of contraindications and drug incompatibilities with which one must be familiar before prescribing. When tolerated, however, this medication can provides significant benefits for trigeminal and glossopharyngeal neuralgias.103,104,105
Today there are several other anticonvulsive drugs that can be useful in neuropathic pain disorders and that have far fewer side effects. Gabapentin (Neurontin), oxcarbazepine (Trileptal), and topiramate (Topamax) are a few of these drugs. Sometimes a combination of anticonvulsive drugs can be used,106 but this is only recommended if a single drug is not adequate to satisfy the patient’s needs.
Neurolytic agents
A neurolytic medication is one that actually destroys a nerve. Neurolytic medications were thought to be very useful in pain management, until the profession began to better understand deafferentation pain. Now we appreciate that destruction of a nerve can actually lead to a pain condition. For this reason, neurolytic agents are rarely used today. The most common neurolytic drug used to destroy peripheral nerves is 95% ethyl alcohol.107 Sometimes phenol is added. Although it is an effective neurolytic, its denervating effect does not preclude regeneration of the peripheral axons and therefore gives only temporary relief.108 The hazards of neuritis, extensive local fibrosis, and deafferentation effects restrict its use in modern practice.
Glycerol has also been used as a neurolytic agent.109,110 The successful use of glycerol (0.30 mL) injected into the retrogasserian space for the treatment of trigeminal neuralgia has been reported.111 About 90% of the patients remained pain-free after one or two injections. About 60% noticed slight numbness, but otherwise there were no significant sensory effects. No dysesthesia or anesthesia dolorosa was observed. It is thought that glycerol acts upon demyelinated axons assumed to be involved in the triggering mechanism of neuralgia.
Uricosuric agents
Antihyperuricemic agents are needed in the treatment of hyperuricemia involving the TMJs. Although its specific action is unknown, colchicine suppresses acute attacks of gout and relieves the pain generated by such attacks. For the treatment of chronic gouty arthritis, probenecid is useful. It is a uricosuric and renal tubule blocking agent that inhibits the reabsorption of urates in the tubules of the kidney. By thus increasing the excretion of uric acid, the serum urate level is lowered. However, probenecid may induce exacerbations of acute gout, however, and should not be used until the acute symptoms are controlled with colchicine. A combination of the two medications is useful in treating chronic gouty arthritis that is complicated by occasional recurrent attacks of acute gout. Gout can involve the TMJ.112,113,114
Dietary considerations
The possibility of dietary manipulation to influence a number of physical complaints, including pain, has come under serious investigation in recent years. Although it is logical that diet would have a significant effect on the patient’s ability to recover from injury or adapt, the data are not impressive. One of the chief relationships between diet and pain that has been investigated is L-tryptophan, which converts into 5HT. Brain and spinal cord serotoninergic neurons are actively involved in nociceptive responses as well as in the analgesic effects of opiates. On the basis of pharmacologic, surgical, electrophysiologic, and dietary manipulative data, it is evident that increased activity of 5HT interneurons is associated with analgesia and enhanced drug potency.115 Hosobuchi et al116 and Schwarz et al117 reported that when patients with chronic pain who were unable to obtain pain relief from 30 mg of IV morphine in divided doses due to drug tolerance were placed on diets supplemented with 4 g of L-tryptophan a day for several weeks, they were able to achieve significant pain relief from opiates. They were also able to lead more active lives, even while reducing their daily opiate intake. Seltzer et al118,119 reported significant elevation of pain tolerance by the dietary supplementation of L-tryptophan. Cheng and Pomeranz120 reported that naloxone-reversible analgesia induced by electroacupuncture was significantly increased when dietary D-phenylalanine and D-leucine were added.
The synthesis of brain 5HT is dependent on the availability of plasma tryptophan, 90% of which is bound to albumin and must compete with other amino acids at the blood-brain barrier.121 It is estimated that only about 2% of plasma tryptophan is hydroxylated by tryptophan hydroxylase to form the precursor to 5HT.122 This conversion requires the presence of adequate vitamin B6.123 Brady et al124 reported that, in a double-blind study, the 10 chronic pain patients included in their study obtained a measure of relief by taking 4 g of L-tryptophan per day and consuming a low-protein, low-fat, high-carbohydrate diet for 8 weeks. Seltzer et al125 reported a reduction in clinical pain and elevated pain tolerance for randomly selected chronic maxillofacial pain patients as the result of 4 weeks of tryptophan supplement therapy.
The dietary considerations in pain therapy, especially for neurovascular pain and fibromyalgia,117,126,127,128,129,130 are becoming more popular. As more is learned about the role of neurotransmitters and protein fractions such as glutamic acid, substance P, glycine, gamma-aminobutyric acid, cholecystokinin, and somatostatin, a variety of dietary manipulations and additional medicinal forms of therapy likely will become available. 131 The dietary approach would not be expected to influence the management of acute pain complaints, however, because of the time factor. Like other nutritional approaches to disease, several weeks are usually required for the effects of dietary therapy to become apparent clinically.
Physical Therapy
Modalities
Physical therapy modalities refer to those treatments that utilize an instrument, device, or agent to accomplish the desired effect. These are sensory stimulation, ultrasound, electrogalvanic stimulation (EGS), and deep heat.
Sensory stimulation
Sensory stimulation can be divided into several categories according to the tissue and mode of stimulation: cutaneous, transcutaneous, and percutaneous.
Cutaneous stimulation. Although the stimulation of skin has been used to obtain relief from pain since ancient times, it has only been during the last few decades that some understanding of the mechanism involved has come to light, along with a revival of many old remedies. The effect occurs through stimulation of thick myelinated cutaneous afferents, A-beta neurons chiefly.132,133 Whether other inhibitory mechanisms are involved remains to be determined.
There are many forms of cutaneous stimulation that effectively attenuate pain. Perhaps the oldest and most natural is pressing or rubbing the skin over the site of injury. Superficial massage is an important means of reducing pain (Fig 9-6). It is enhanced by adding a stimulating substance such as alcohol or menthol ointment.
Counterirritation is an age-old pain remedy. 134 Mustard plasters can still be remembered by many. It is well known that mild stimulation of nociceptors also increases pain-inhibitory mechanisms. A mixture of aconite and iodine has been used for this purpose by dental clinicians. Currently, vapocoolant therapy is important in relieving myofascial trigger point pain (Fig 9-7). Initially thought to be effective through its local anesthetic action, it is now recognized that the vapocoolant mildly stimulates the cutaneous nociceptors as well as the thicker A-beta fibers. Thus, the body’s pain inhibitory system is activated. As long ago as 1902, ethyl chloride was strayed on the eardrum to alleviate the pain of otitis media.135 Kraus136 introduced the use of ethyl chloride spray for the treatment of painful motion in 1941. Travell advocated its use in 1949 for the treatment of somatic trigger areas137 and in 1952 for treating skeletal myospasms.138 Schwartz139 applied it to the treatment of painful mandibular movement in 1954.

Fig 9-6 Gentle massage of the painful area can often reduce pain. The reduction of pain may be due to gate-control mechanisms. Deep massage can be used for musculoskeletal pains and may provide pain relief by altering the local tissue status.
Intermittency is an essential element in vapocoolant therapy and probably is the reason why it is more effective than are other forms of thermal therapy. Thick afferents adapt quickly to stimulation and therefore are more reactive to gradient changes. The application of heat and cold to obtund pain is very old. It has long been known to be more effective when the heat and cold are applied alternately for brief periods (Fig 9-8). Infrared heat is another form of cutaneous stimulation.
The use of mechanical vibration to relieve pain is an old pain remedy. It has been reported that at least one third of patients obtain complete relief from dental pain by this method, and the period of relief outlasts the stimulation by several hours.140,141,142,143 Mechanical vibration also enhances the effect of transcutaneous electrical stimulation in about half the subjects.144 Lundeberg 145 reported that in a study of 267 patients with chronic neuropathic or musculoskeletal pain treated with vibratory stimulation, 59% achieved more than 50% relief.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses