Chapter 8 The immune system and the oral cavity
The immune system: general considerations
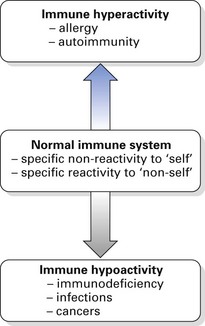
There are two kinds of immunological defence:
These concepts are summarized in Figure 8.1.
The innate immune system
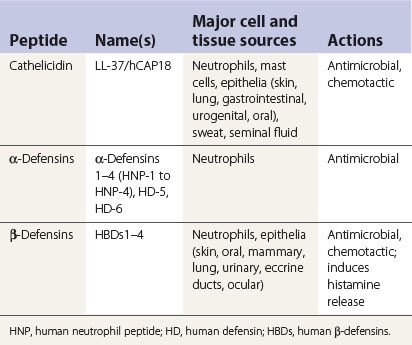
Defensins and cathelicidins
Defensins and cathelicidins are two major families of mammalian antimicrobial proteins. They contribute to host innate antimicrobial defences by disrupting the integrity of the bacterial cell membrane. Further, several members of defensins and cathelicidins have been shown recently to have chemotactic effects on host cells. Their capacity to mobilize various types of phagocytic leukocytes, immature dendritic cells and lymphocytes, together with their other effects, such as stimulating interleukin-8 production and mast cell degranulation, provides evidence for their participation in alerting, mobilizing and amplifying innate and adaptive antimicrobial immunity of the host (Table 8.1). In brief, upon microbial invasion, epithelial cells/keratinocytes and tissue macrophages are induced to produce β-defensins (especially HBD2 and 3) and cathelicidin/LI-37. The defensins and cathelicidin form gradients that, in tandem with other chemotactic mediators (e.g. chemokines), lead to extravasation of various types of leukocytes to the site of infection in order to overcome the invading pathogens (Table 8.2).
Table 8.1 Antigen-non-specific defence chemicals in oral secretions
| Chemical | Antimicrobial function(s) | Major cell source(s) |
|---|---|---|
| Calprotectin | Divalent cation chelator, restricts microbe nutrition | Oral epithelial cells and neutrophils |
| Defensins (α and β types) | Membrane pore-forming peptides, cause osmotic lysis | Leukocytes and epithelial cells |
| Cathelicidins | Lysosomal antimicrobial polypeptides | Macrophages and neutrophils |
| Saliva | Ig, lysozyme, lactoferrin, peroxidases and GCF | Salivary acinar cells |
| Lysozyme | Muramidase activity, aggregates microbes and amphipathic sequences | Macrophages, epithelial cells and neutrophils |
| Peroxidase | Oxidizes bacterial enzymes in glycolytic pathways | Salivary acinar cells, neutrophils, eosinophils |
| His-, Cis- statins | Various effects | Salivary acinar cells |
| SLPI, PRP | Antiviral activities | Various cell types |
| GCF | Provides blood components | Various cell types |
| Mucins | Aggregates bacteria, various effects, homotypic and heterotypic complexes | Salivary acinar cells |
SLPI, secretory leukocyte protease inhibitor; PRP, proline-rich proteins; GCF, gingival crevicular fluid; Ig, immunoglobulin.
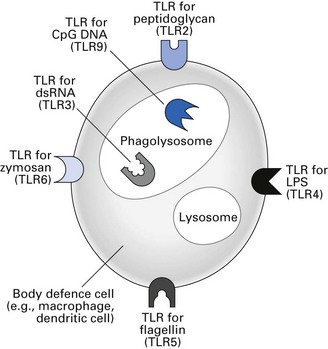
Pathogen-associated molecular patterns, pattern-recognition receptors and Toll-like receptors
Binding of PAMPs to signalling PRRs promotes the synthesis and secretion of regulatory molecules such as cytokines that are crucial to initiating innate immunity. Various types of TLRs bind different PAMPs and initiate different types of innate immune responses (Fig. 8.2). PAMPs can also be recognized by a series of soluble PRRs in the blood that function as opsonins and initiate the complement pathway.
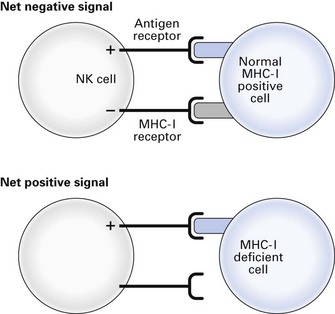
Natural killer cells
Natural killer (NK) cells are non-phagocytic lymphocytes that account for up to 15% of blood lymphocytes and have a special role in the killing of virus-infected and malignant cells (Fig. 8.3). These cells have two kinds of receptors with opposing action: antigen receptors able to recognize specific molecules on target cells, through which activation signals are transmitted, and receptors that recognize self major histocompatibility complex I (MHC I) antigens (see below) through which inactivation signals are transmitted. Activation of NK cells can only occur when there is no inactivation signal, so virus-infected and tumour cells with downregulated MHC I antigens are susceptible to NK cytotoxicity, but normal MHC I-positive cells are protected. The killing mechanism is activated by cytokines released by virus-infected cells, tissue cells, lymphocytes and NK cells themselves. The NK cells are also important in the adaptive immune response, being the effector cells for killing antibody-coated microorganisms.
Acute-phase proteins
Alternative activation
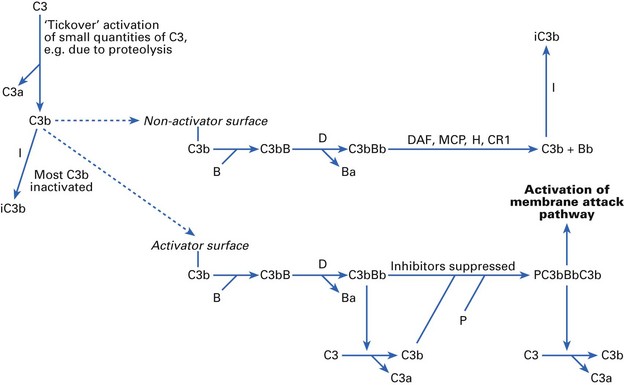
Complement factor C3 is the central component of both the classical and alternative pathways (Fig. 8.4). Products of C3 activation, C3b and inactivated C3b (iC3b) bind to microorganisms and are recognized by complement receptors (CRs) on phagocytes. If any C3b molecules bind to a normal host cell surface, they can then bind the next component in the sequence, factor B. Factor D (the only complement factor present in body fluids as an active enzyme) splits off a small fragment, Ba, leaving an active C3 convertase, C3bBb, on the cell surface. However, the normal host cell is able actively to dissociate and inactivate C3bBb. This is achieved by the concerted action of regulatory proteins decay-accelerating factor (DAF), membrane cofactor protein (MCP), β1H globulin (factor H), CR1 and factor I.
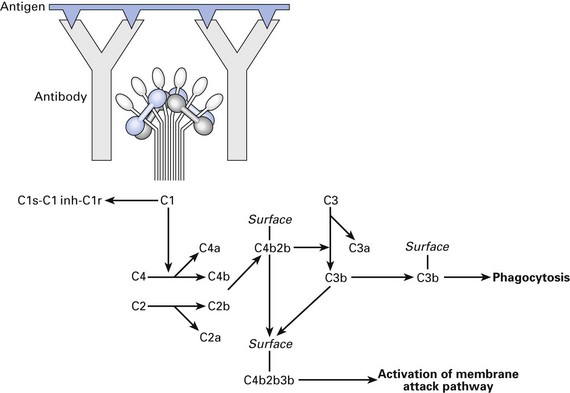
Classical activation
Classical pathway of complement activation (Fig. 8.5) is mainly initiated by complexes of antigen with antibody. Antibodies of the immunoglobulin (Ig) IgG1, IgG2, IgG3 and IgM classes, but not IgG4, IgA, IgD or IgE, can activate the classical pathway.
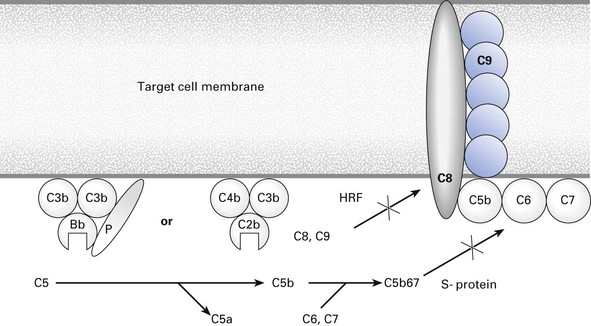
Membrane attack
The peptides Bb and C2b, bound into their respective alternative (PC3bBbC3b) and classical (C4b2b3b) pathway enzymatic complexes, initiate membrane attack (Fig. 8.6) by splitting a small peptide, C5a, from C5 to form C5b. This molecule binds C6 and C7. Cell-bound C5b67 acts as a template for the binding of one molecule of C8 and up to 18 molecules of C9. Normal cells in the body are largely protected from bystander lysis by homologous restriction factor (HRF), which intercepts C8 and C9 before they can be properly assembled into the membrane attack complex (MAC). The MAC, with a molecular weight of 1–2 × 106, forms transmembrane channels, which permit osmotic influx so that the target cell swells up and bursts.
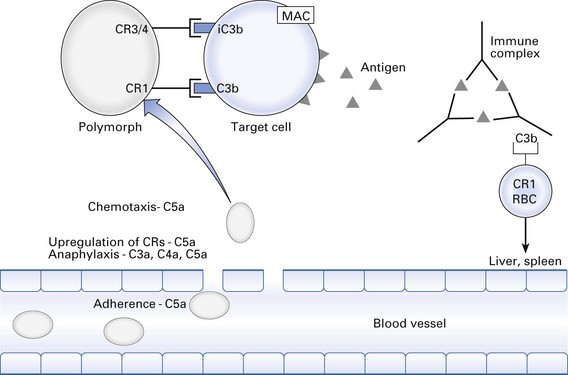
Biological effects of complement activation
Probably the most important function of the complement system is to opsonize antigen–antibody (immune) complexes, microorganisms and cell debris for phagocytosis (Fig. 8.7). This is achieved by deposition of C3b and iC3b on the particle. Phagocytes bind to the particle via CR1, CR3 and CR4. Also, CR1 is found on erythrocytes, which can bind immune complexes coated with C3b and transport them to the spleen or liver for digestion by macrophages.
Cells of the immune system
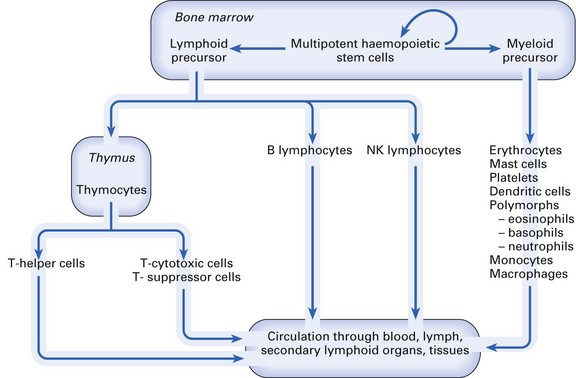
All the cells of the immune system (Fig. 8.8) are derived from self-regenerating haematopoietic stem cells present in bone marrow and foetal liver. These differentiate along either the myeloid or the lymphoid pathway. Myeloid precursor cells give rise to mast cells, erythrocytes, platelets, dendritic cells, polymorphs (eosinophils, basophils, neutrophils) and mononuclear phagocytes (monocytes in the blood, macrophages in the tissues). Lymphoid precursor differentiation gives rise to T (thymus-dependent) lymphocytes, B (bone marrow-derived) lymphocytes and NK lymphocytes.
The lymphoid organs
Mature lymphoid cells continuously circulate between the blood, lymph, lymphoid organs and tissues until they encounter an antigen, which will cause them to become activated (see Chapter 9).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses