8
Healing
Now that the battle has been won, there is a lot of mopping up and repair work for the troops to do. As with any army, some soldiers get right to work, while others will goof off until much of the work is done. Right up there in the front ranks are the macrophages with their brooms at the ready, and just in case any of the enemy are still around, neutrophils are still on patrol. The construction workers (fibroblasts and endothelial cells) will get started as soon as the mess is cleaned up.
Healing includes the processes of resolution, regeneration, and repair.
The events of healing occur somewhat later than the initial steps of inflammation, but there is a considerable amount of overlap. For example, phagocytosis is a defensive reaction, but it is an important early event in the reparative process. Similarly, fibroplasia is a fundamental process in both chronic inflammation and repair.
One of the primary functions of the inflammatory process is to heal wounded tissue. Healing may occur as a result of either resolution or repair. Resolution can only occur if regeneration is possible.
Fibrous repair
The alternative to resolution is fibrous repair—repair by the formation of granulation tissue. This involves proliferation of new small blood vessels (neovascularization) and fibroblasts. An overview of the stages of repair follows.
Wound repair signals
- Growth factors (GFs)
- Cell–matrix interactions
- Cell–cell interactions
Scientists have so far found about two dozen different growth factors that participate in healing. There are likely to be others.
When a GF binds to its receptor, the receptor becomes transiently activated. This activation causes, in turn, the activation of other proteins in the growth-stimulatory pathway and the production of a variety of small regulatory molecules called second messengers (Fig 8-1). G proteins translate and integrate external signals for the cell’s second messengers. Some of these second messengers are ultimately transmitted to the nucleus, where the expression of specific genes is induced. GFs stimulate cells in a number of ways:
- Act as mitogens to stimulate cells to proliferate
- Induce differentiation
- Stimulate synthesis and secretion of proteins
- Facilitate attachment of the cell
- Alter the shape of the cell
- Stimulate the cell to migrate
In the following list and in Table 8-1 are examples of some of the better known growth factors:
Platelet-derived growth factor (PDGF) is stored in platelet granules and is also produced by activated macrophages, endothelium, smooth muscle, and some tumor cells. It causes migration and proliferation of fibroblasts, smooth muscle cells, and monocytes.
Epithelial growth factor (EGF) has been shown to accelerate the rate of healing of partial-thickness skin wounds in humans. It is mitogenic for epithelial cells and fibroblasts.
Fibroblast growth factors (FGFs) are a family of growth factors capable of stimulating chemotaxis and mitogenesis in fibroblasts, endothelial cells, and smooth muscle. Basic FGF is secreted by activated macrophages and is present in many organs whereas acidic FGF is confined to neural tissue.
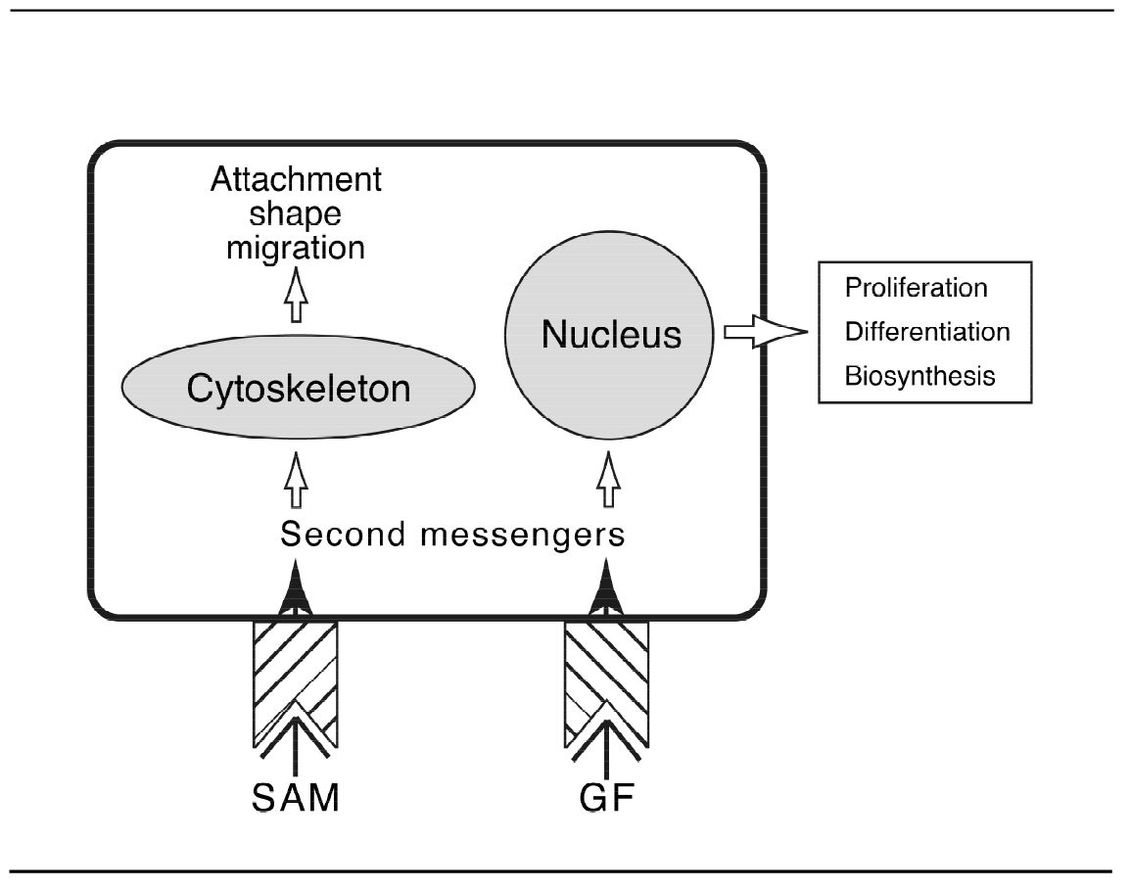
Fig 8-1 Activation of second messengers in a cell by substrate adhesion molecules (SAM) and growth factors (GF).
Vascular endothelial growth factor (VEGF) evokes new blood vessel formation. This involves basement membrane degradation, endothelial cell migration, proliferation, differentiation, and capillary tube formation.
Transforming growth factor-alpha (TGF-α) binds to EGF receptors and produces many of the same biologic effects as EGF (eg, accelerates epidermal regeneration).
Transforming growth factor-beta (TGF-β) can be produced by different cell types including endothelium, T cells, macrophages, and platelets. It influences (usually inhibits) the rate of proliferation of many cell types. It stimulates fibrogenesis by inducing fibroblast chemotaxis, increases production of collagen and fibronectin, and inhibits collagen degradation.
IL-1 and TNF are chemotactic for fibroblasts and stimulate collagen synthesis.
Table 8-1 Major growth factors in wound healing
| Function | Mediators |
|---|---|
| Fibroblast proliferation | PDGF, EGF, FGF, IL-1/TNF |
| Fibroblast migration | PDGF, EGF, FGF, IL-1/TNF, TGF-β |
| Chemotactic for macrophages | PDGF, FGF, TGF-β |
| Neovascularization | VEGF, FGF |
| Collagen synthesis | TGF-β, PDGF, TNF |
| Collagen secretion | PDGF, FGF, EGF, TNF |
Cell–matrix interaction
The extracellular connective tissue consists of basement membrane, as well as the various types of collagen, elastin, and proteoglycans. Basement membranes contain the glycoproteins fibronectin and laminin, as well as collagen types IV and V.
Extracellular matrix proteins, often referred to as substrate adhesion molecules (SAMs) interact with cell surface receptors to induce intracellular signals (second messengers). These signals influence cell attachment, shape, migration, proliferation, differentiation, and biosynthesis (see Fig 8-1).
The best studied proteins of the extracellular matrix are the fibronectins, a family of glycoproteins. The molecule consists of domains, which bind to fibrin, heparan sulfate, and cell surfaces. Other matrix proteins include tenascin; cytotactin; laminin; collagen types IV, V, and VII; and heparan sulfate proteoglycan.
Fibronectin or fibronectin fragments promote migration of endothelial cells and fibroblasts into an area of injury.
Laminin is the most abundant glycoprotein in basement membranes. It contains binding domains for heparan sulfate, type IV collagen, and cells.
Cells contain surface membrane proteins, called integrins, which are specific transmembrane receptors for matrix proteins. They recognize the specific amino acid sequence of the tripeptide arginine-glycine-aspartic acid found in these proteins. This sequence is thought to play a key role in cell adhesion.
Integrins include receptors for fibronectin, collagen, and laminin, as well as glycoproteins on the surface of platelets, and receptors for leukocyte adhesion molecules.
It is thought that integrins interact with the cell cytoskeleton and initiate the producti/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


