Chapter 7
Topical Disinfectants for Root Canal Irrigation
Bettina Basrani
Discipline of Endodontics, University of Toronto, Toronto, ON, Canada
Markus Haapasalo
Department of Oral Biological and Medical Sciences, Unreality of British Columbia, Vancouver, BC, Canada
Introduction
Bacteria have long been recognized as the primary etiologic factors in the development of pulp and apical lesions (1). Successful root canal therapy depends on the thorough chemomechanical debridement of pulpal tissue, dentin debris, and infective microorganisms (2). For a treatment to reach favorable outcomes in endodontic infection management, the recognition of the problem and the removal of the etiological factors is important.
Irrigation is defined as “to wash out a body cavity or wound with water or a medicated fluid” and aspiration as “the process of removing fluids or gases from the body with a suction device.” Disinfectant, on the other hand, is defined as an agent that destroys or inhibits the activity of microorganisms that cause a disease (3).
The objectives of irrigation in endodontics are mechanical, chemical, and biological. The mechanical and chemical objectives are as follows: (i) flush out debris, (ii) lubricate the canal, (iii) dissolve organic and inorganic tissue, and (iv) prevent the formation of a smear layer during instrumentation or dissolve it once it has formed. The biological function of the irrigants is related to their antimicrobial effect, more specifically: (i) they have a high efficacy against anaerobic and facultative microorganisms in their planktonic state and in biofilms, (ii) they have the ability to inactivate endotoxin, and (iii) they are nontoxic when they come in contact with vital tissues, are not caustic to periodontal tissues, and have little potential to cause an anaphylactic reaction (4). The characteristics of an ideal irrigant and the classification of current irrigants are shown in Tables 7.1 and 7.2 respectively.
Table 7.1 Characteristics of an ideal endodontic irrigant (5–7).
|
Grossman and Meiman (1941) (5). Reproduced with permission of Elsevier.
Table 7.2 Classification of current irrigants.
|
Irrigation solution in endodontics
Sodium hypochlorite (NaOCl)
Sodium hypochlorite is a chemical compound with the formula NaOCl. Sodium hypochlorite solution, commonly known as bleach, is frequently used as a disinfectant or a bleaching agent. It is the medicament of choice during root canal treatments following its efficacy against pathogenic organisms and pulp digestion. The main characteristics of sodium hypochlorite are summarized in Table 7.3.
Table 7.3 Summary of the characteristics of NaOCl.
|
| Limitations: |
|
History
Hypochlorite was first produced in 1789 in Javelle, France, by passing chlorine gas through a solution of sodium carbonate. The resulting liquid, known as Eau de Javelle or Javelle water, was a weak solution of sodium hypochlorite. However, this process was not very efficient and alternative production methods were sought. One such method involved the extraction of chlorinated lime (known as bleaching powder) with sodium carbonate to yield low levels of available chlorine. This method was commonly used to produce hypochlorite solutions for use as a hospital antiseptic, which was sold under the trade names “Eusol” and “Dakin’s solution.” Sodium hypochlorite as a buffered 0.5% solution was recommended for the irrigation of wounds during World War I by Dakin.
Mode of action
When hypochlorite contacts tissue proteins, within a short time, nitrogen, formaldehyde, and acetaldehyde are formed. The peptide links are broken up to dissolve the proteins. During this process, hydrogen in the imino groups (–NH–) is replaced by chlorine (–N·Cl–), forming chloramines, which plays an important role in antimicrobial effectiveness. Thus, the necrotic tissue and pus are dissolved and the antimicrobial agent can better reach and clean the infected areas. In addition to its application as a root canal irrigant, NaOCl is commonly used to deproteinize hard tissues for biomedical applications.
Estrela (8) reported that sodium hypochlorite exhibits a dynamic balance. Sodium hypochlorite acts as an organic and fat solvent that degrades fatty acids and transforms them into fatty acid salts (soap) and glycerol (alcohol), thus reducing the surface tension of the remaining solution (saponification reaction) (Figure 7.1).
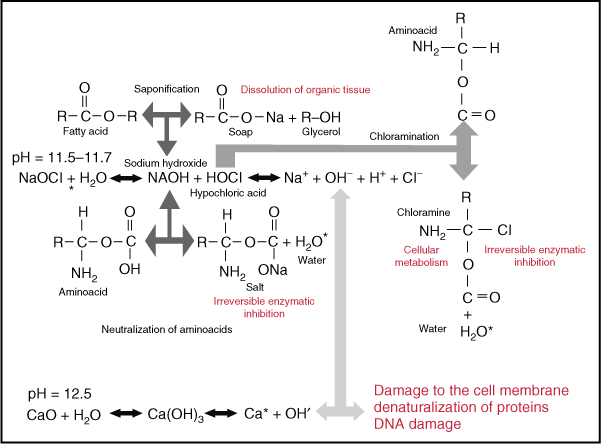
Figure 7.1 Mechanism of the action of NaOCl.
(Courtesy of Dr. Manzur.)
Sodium hypochlorite neutralizes amino acids, forming water and salt (neutralization reaction). With the exit of hydroxyl ions, there is a reduction of pH. When chlorine dissolves in water and gets in contact with organic matter, it forms hypochlorous acid. It is a weak acid with the chemical formula HClO. HClO is an oxidizer. This acid acts as a solvent, releasing chlorine that combined with the protein amino group forms chloramines (chloramination reaction). Hypochlorous acid (HOCl−) and hypochlorite ions (OCl−) lead to amino acid degradation and hydrolysis.
The chloramination reaction between chlorine and the amino group (NH) forms chloramines that interfere in cell metabolism. Chlorine, a strong oxidant, presents antimicrobial action by inhibiting bacterial enzymes, leading to the irreversible oxidation of SH groups (sulfhydryl group) of essential bacterial enzymes (8).
Sodium hypochlorite is a strong base (pH > 11). The antimicrobial effectiveness of sodium hypochlorite, based in its high pH (hydroxyl ions action), is similar to the mechanism of action of calcium hydroxide. The high pH of sodium hypochlorite interferes in the cytoplasmic membrane integrity with an irreversible enzymatic inhibition, biosynthetic alterations in cellular metabolism, and phospholipid degradation observed in lipidic peroxidation (8).
Concentrations
As an endodontic irrigant, NaOCl is used in concentrations between 0.5% and 6%. There has been controversy over the use of different concentrations of sodium hypochlorite during root canal treatment. Some in-vitro studies have shown that NaOCl in higher concentrations is more effective against Enterococcus faecalis and Candida albicans (9–11). In contrast, clinical studies have indicated both low and high concentrations to be equally effective in reducing bacteria from the root canal system (2, 12). NaOCl in higher concentrations has a better tissue-dissolving ability (13); however, even in lower concentrations when used in high volumes it can equally be effective (14, 15). Higher concentrations of NaOCl are more toxic than lower concentrations (16); however, because of the confined anatomy of the root canal system, higher concentrations have successfully been used during root canal treatment, with a low incidence of mishaps. Altogether, if lower concentrations are to be used for intracanal irrigation, it is recommended that the solution be used in higher volume and in more frequent intervals to compensate for the limitations of low concentrations (15).
Instrumentation coupled with an antimicrobial irrigant, such as NaOCl, has been shown to yield more negative cultures than instrumentation alone (17–19). However, even with the use of NaOCl, removal of bacteria from the root canal systems following instrumentation remains an elusive goal.
Grossman (20), observing pulp tissue dissolution capacity, reported that 5% sodium hypochlorite dissolves this tissue in between 20 min and 2 h. The dissolution of bovine pulp tissue by sodium hypochlorite (0.5%, 1.0%, 2.5%, and 5.0%) was studied in-vitro under different conditions (8). It was concluded that (i) the velocity of dissolution of the bovine pulp fragments was directly proportional to the concentration of the sodium hypochlorite solution and was greater without the surfactant; (ii) the variation of surface tension, from the beginning to the end of pulp dissolution, was directly proportional to the concentration of the sodium hypochlorite solution and was greater in the solutions without surfactant. Solutions without surfactant presented a decrease in surface tension and those with surfactant an increase; (iii) with the elevation of temperature of the sodium hypochlorite solutions, dissolution of the bovine pulp tissue was more rapid; (iv) the percent variation of the sodium hypochlorite solutions, after dissolution, was inversely proportional to the initial concentration of the solution, or, in other words, the greater the initial concentration of the sodium hypochlorite solutions, the smaller the reduction of its pH (8).
Time of exposure for optimal effect
There is considerable variation in the literature regarding the antibacterial effect of NaOCl (21). In some articles, hypochlorite is reported to kill the target microorganism in seconds, even at low concentrations, although other articles published report considerably longer times for the killing of the same species (21). Such differences are a result of the confounding factors in some of the studies. In the experiments, it was found that the presence of organic matter has a great effect on the antibacterial activity of NaOCl. Haapasalo and colleagues (21) showed that the presence of dentin caused marked delays in the killing of E. faecalis by 1% NaOCl. Many of the earlier studies were performed in the presence of an unknown amount of organic matter. When the confounding factors are eliminated, it has been shown that NaOCl kills the target microorganisms rapidly even at low concentrations of less than 0.1% (22, 23). However, in vivo, the presence of organic matter (inflammatory exudate, tissue remnants, and microbial biomass) consumes NaOCl and weakens its effect. Therefore, continuous irrigation and time are important factors for the effectiveness of NaOCl (21).
In summary, even fast-acting biocides such as NaOCl require an adequate working time to reach their potential. Chlorine, which is responsible for the dissolving and antibacterial capacity of NaOCl, is unstable and is consumed rapidly during the first phase of tissue dissolution, probably within 2 min (14); therefore, continuous replenishment is essential. This should especially be considered in view of the fact that rotary root canal preparation techniques have expedited the shaping process. The optimal time that a hypochlorite irrigant at a given concentration needs to remain in the canal system is an issue yet to be resolved (24).
Storage and handling
The following points should be considered when handling sodium hypochlorite:
- stability of NaOCl solutions is reduced by lower pH, presence of metallic ions, exposure to light, open containers, and higher temperatures.
- ensure good shelf life, all solutions should be stored in light-proof (opaque glass or polythene), airtight containers, in a cool place.
- diluted, it should be done as soon as possible after purchase, because dilute solutions deteriorate less rapidly than concentrated solutions.
- bleach solutions produced and stored in this manner will deteriorate more rapidly than Milton, because they do not have the added salt, which provides stability.
- undiluted bleach is used, the bottle should always be tightly sealed, and the bleach should be discarded by the “use by” date. The same goes for Milton as well; as long as the container and lid are intact, the product will be effective until the expiry date.
- opening of a container or failure to close it securely would have an effect similar to leaving a container open, and the shelf life would be reduced accordingly.
- containers should never be used for sodium hypochlorite, as the hypochlorite will react with the metal in the containers.
- corrosive nature of sodium hypochlorite must be considered before disposal. As drainage pipes from sinks and dental units may use stainless steel, copper, galvanized steel, poly(vinyl chloride) (PVC), polythene, and perhaps other materials, copious quantities of water should be flushed down all drains at the time of disposal to avoid risk of perforation of drainage traps that have undiluted sodium hypochlorite in them for any period (25).
Safety
Sodium hypochlorite is a nonspecific oxidizing agent. Products of the oxidation reactions are corrosive. Solutions burn skin and cause eye damage, particularly when used in concentrated forms. However, as recognized by the National Fire Protection Association (NFPA), only solutions containing more than 40% sodium hypochlorite by weight are considered hazardous oxidizers. Solutions less than 40% are classified as a moderate oxidizing hazard (NFPA 430, 2000). The toxic effects of NaOCl on vital tissues include hemolysis, epithelial ulceration, and necrosis (4).
Several mishaps during root canal irrigation have been described in the dental literature. These range from damage to the patient’s clothing, splashing the irrigant into the patient’s or operator’s eye, injection through the apical foramen, and allergic reactions to the irrigant, to inadvertent use of an irrigant as an anesthetic solution (4). Preventive measures that should be taken to minimize potential complications with sodium hypochlorite are presented in Table 7.4 (26) (Figure 7.2).
Table 7.4 Protective measures during NaOCl irrigation.
|
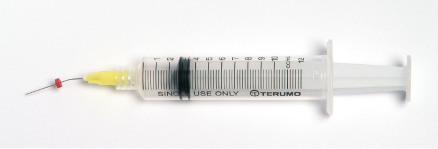
Figure 7.2 Placement of rubber stopper on irrigation needle to prevent NaOCl accident.
A literature review of inadvertent extrusion of NaOCl beyond the apical foramen found similar symptoms, regardless of the concentration, with tissue responses proportional to the volume of NaOCl extruded (27) (Figure 7.3). Extrusion of NaOCl into the apical tissues can result from several pathways. A wide apical foramen, lack of apical constriction, or extreme pressure might all lead to the extrusion of NaOCl. Most complications occur because of the incorrect working length, widening of the apical foramen, lateral perforation, or binding of the irrigating needle (28).
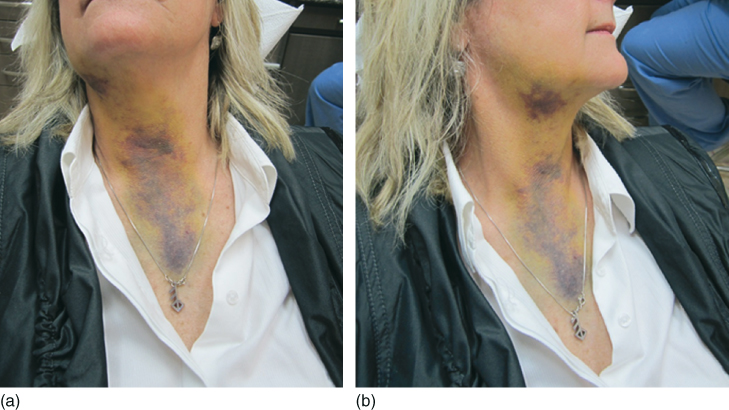
Figure 7.3 (a,b) NaOCl accident.
The main symptoms when injected into the apical tissues are immediate severe pain, immediate edema of the neighboring soft tissues, possible extension of edema over the injured side of the face, upper lip and infraorbital region, profuse bleeding from the root canal, profuse interstitial bleeding with hemorrhage of the skin and mucosa (ecchymosis), chlorine taste and irritation of the throat after injection into the maxillary sinus, secondary infection, reversible anesthesia, or paresthesia.
Current treatment protocols for NaOCl accidents have been determined mainly from the numerous case reports published, rather than more evidence-based research efforts. Mehdipour et al. (29) suggest early recognition of extrusion, immediate canal irrigation with normal saline, encouragement of bleeding, pain control with local anesthetics and analgesics, and warm compresses and frequent warm mouth rinses for the stimulation of the local systemic circulation, reassurance of the patient, and monitoring the improvement. Cancellous bone is significantly affected by NaOCl, whereas cortical bone is minimally affected. Cancellous bone after a NaOCl accident is less dense, with broken and dissolved architecture. The deeper penetration of the test needle is interpreted as the result of a disrupture in the structural integrity of the cancellous bone. The principal harm is to the cells, because they are dependent on the specific fluid environment in which they are found; NaOCl changes that environment, causing cellular necrosis and apoptosis. The damaged matrix can then become a nidus for infection. Trabecular bone is damaged by the toxic effects of NaOCl. The less cellular cortical bone is clearly less affected. The results show that the loss of organic content of the bone and demineralization are significant, and no sign of living cellular content remains (30).
Effect of NaOCl on dentin
Dentin is composed of approximately 22% organic material by weight. Most of this consists of type I collagen, which contributes considerably to the mechanical properties of dentin (31). Sodium hypochlorite is known to fragment long peptide chains and to chlorinate protein terminal groups; the resulting N-chloramines are broken down into other species (32). Consequently, hypochlorite solutions may affect mechanical dentin properties via the degradation of organic dentin components (33).
A study on bovine dentin suggested that within the time frame of a root canal treatment, concentrated hypochlorite solutions cause untoward effects on dentin biomechanics (34). A 2 h exposure of dentin to NaOCl solutions of more than 3% (w/v) significantly decreases the elastic modulus and flexural strength of human dentin compared to physiological saline (35). However, contrasting results have also been published (36). A recent study showed a clear concentration-dependent effect of NaOCl solutions on the mechanical dentin properties resulting from the disintegration of the organic dentin matrix (37) (Figure 7.4).
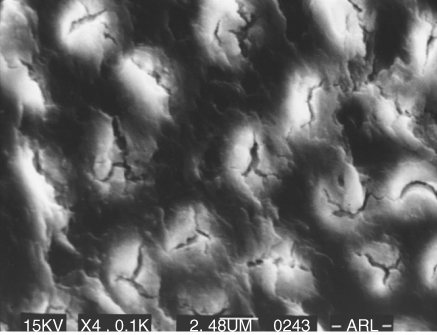
Figure 7.4 Dentin treated with NaOCl.
There have been several reports of the adverse effects of sodium hypochlorite on physical properties such as flexural strength, elastic modulus, and microhardness of dentin. These changes in the physical properties of dentin come not only from changes in the inorganic phase but also from the organic phase of dentin. Moreover, in their eagerness to ensure “complete” disinfection, dentists vary not only the concentration but also the volume, duration, flow rate, and temperature in their attempts to eliminate all bacteria (37). It is quite clear from the literature that the higher the concentration of sodium hypochlorite, the greater the deleterious effects on dentin. These effects include the reduction of the elastic modulus and the flexural strength.
Sodium hypochlorite penetration of dentinal tubules
Not many studies have analyzed the penetration of NaOCl inside the dentinal tubules. Zou et al. (38) are the first to report hypochlorite penetration into dentin measured with such accuracy (micrometers). Within their experimental setup, the depth of hypochlorite penetration varied between 77 and 300 µm. The three parameters potentially affecting hypochlorite penetration that were evaluated in the present study were concentration, time, and temperature. All of these did have an impact on the penetration, but the effect was generally less than anticipated. Perhaps the most surprising observation was that increasing the concentration from 1% to 6% did not result in more than 30–50% increase in penetration. Longer exposure time in the present study resulted in deeper penetration of hypochlorite, although the speed of penetration declined sharply over time. For example, at 20 °C, penetration depth of 1% NaOCl in 2 min was about 77 µm; after another 18 min at the same temperature, the depth reached about 185 µm. Because the solubilizing abilities of NaOCl solutions are reduced by contact with organic material, it can be speculated that most of its activity is lost after 2 min, and continuous replenishment of fresh solution will be needed. The antibacterial effectiveness of NaOCl is dependent on its concentration, temperature, and volume and contact time in the root canal. The results showed that the three variables all had an effect on NaOCl penetration, but the effect was not very pronounced for any of the factors alone. The penetration depths of 1%, 2%, 4%, and 6% solutions after 2 min at room temperature were 77, 96, 105, and 123 µm, respectively. The highest values, 291 and 300 µm, were found in the groups treated by 6% NaOCl at 37 and 45 °C for 20 min. Within the limitations of this study, temperature, time, and concentration all play a role in determining the depth of hypochlorite penetration into dentinal tubules. Deepest penetration was obtained when these factors were present simultaneously, suggesting an additive effect (Figure 7.5) (38).
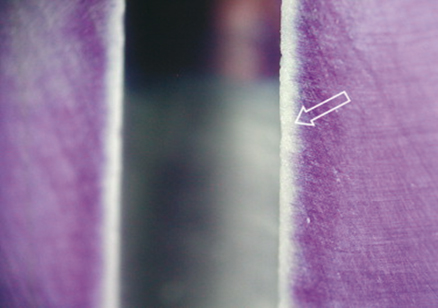
Figure 7.5 Sodium hypochlorite penetration of dentinal tubules.
Allergic reactions to NaOCl
Although few reports on allergy-like reactions to sodium hypochlorite have been published (39, 40), real allergies to sodium hypochlorite are unlikely to occur as both sodium and chlorine are essential elements in the physiology of the human body. Nevertheless, hypersensitivity and contact dermatitis may occur in rare cases. In cases of hypersensitivity against sodium hypochlorite, chlorhexidine (CHX) should not be used because of the chlorine content. In such cases, the use of an alternative irrigant with high antimicrobial efficacy such as iodine–potassium iodide (IPI) should be considered. Before use, any allergy against iodine must be ruled out. Further irrigants such as alcohol or tap water are less effective against microorganisms and do not dissolve vital or necrotic tissue. Calcium hydroxide could be used as a temporary medicament as it dissolves both vital and necrotic tissues (41, 42).
Effect on biofilm
Biofilm growth over root surfaces has been demonstrated on teeth with chronic apical periodontitis and teeth refractory to root canal treatment. Bacteria organized as biofilms have been found in inaccessible areas of necrotic pulp space and on root surfaces and cemental lacunae. Growing within a competitive environment, the organisms within biofilm generally have a low metabolic rate and tend to be very resistant to antimicrobial substances. The negatively charged polymers within the matrix may neutralize strong oxidizing agents, making it difficult for them to penetrate and kill microorganisms. Because of the close proximity of bacterial cells within the biofilm, DNA exchange readily takes place and can rapidly transfer antibiotic resistance. Therefore, antimicrobial substances that easily kill free-floating organisms have not shown the same effectiveness on the same organisms originating from a biofilm. In addition, the structure of biofilm offers protection to resident bacteria from immune defenses. These properties of biofilm help to explain the chronic nature and resistance of some endodontic infections (43).
Clegg et al. (44), in their classic paper on the effect of NaOCl on biofilms, reported that 6% NaOCl was the only agent capable of both physically removing artificial biofilm and killing bacteria. There was a dose-dependent effect of NaOCl against bacteria, as higher concentrations were more antibacterial. This confirms the results of previous studies that also demonstrated the concentration-dependant antibacterial nature of NaOCl. One percent and 3% NaOCl showed some disruption and physical removal of bacteria when viewed with the scanning electron microscopy (SEM); however, both gave positive cultures when their dentinal shavings were cultured, indicating that bacteria had escaped the effects of the irrigant probably by invading the dentinal tubules. The lower the concentration of NaOCl, the better the chances for more bacteria survival. However, the lower NaOCl concentrations may have been more effective against bacteria if they were replenished or given additional time to exert their antimicrobial properties. The antibiofilm effects of NaOCl may be a result of the removal of organic tissue, eliminating the bacterial attachment to dentin and other organisms (44).
A recent study evaluated the antibacterial effect of a several irrigating solution on 3-week-old E. faecalis biofilms. The agents analyzed were QMiX (see the details below in this chapter), 2% CHX, MTAD, 1%, and 2% NaOCl. QMiX and 2% NaOCl killed up to 12 times more biofilm bacteria than 1% NaOCl (P < 0.01), 2% CHX (P < 0.05; P < 0.001), and MTAD (P < 0.05; P < 0.001) (Figure 7.6) (45).
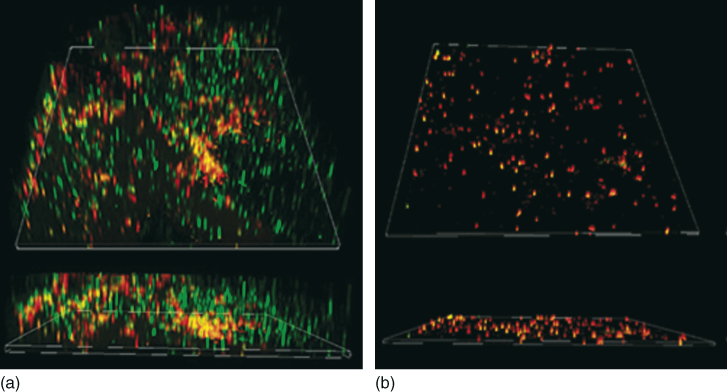
Figure 7.6 The three-dimensional confocal laser scanning microscopy reconstruction of the in-vitro 7 days’ biofilm of Enterococcus faecalis subjected to antimicrobial irrigants (inlet shows the sagittal section). These biofilms were stained using Live/Dead BacLight stain (green: viable cell and red: dead cell) (a) the biofilm receiving no treatment, (b) the biofilm exposed to sodium hypochlorite. The biofilm structure in the sodium hypochlorite treated specimen is disrupted.
(Courtesy of Dr. Anil Kishen.)
Increasing the efficacy of NaOCl
Possible ways to improve the efficacy of hypochlorite preparations in tissue dissolution are to increase the pH and the temperature of the solutions, use ultrasonic activation (7), and extend the working time.
Increasing the temperature of sodium hypochlorite
Cunningham (46) and Joseph reported that the collagen-dissolving ability of 2.6% sodium hypochlorite was comparable to that of 5.25% at both 21 and 37 °C. The investigators also compared the solutions’ ability to kill bacteria at different temperatures. They tested the ability of 2.6% and 5.25% sodium hypochlorite in reducing a planktonic culture of Escherichia coli to below culturable level at 20 and 37 °C. They found that it took less time to kill E. coli in both concentrations at 37 °C. Interestingly, it was also reported that increasing the temperature of sodium hypochlorite to 50 °C did not help in making the root canal cleaner. However, at the higher temperature (50 °C), Berutti et al. (47) observed a thin, less organized, and less adherent smear layer on the root canal wall. This thinner layer was not evident on root canals irrigated with sodium hypochlorite at 21 °C.
From the above studies, it is apparent that raising the temperature may have some benefit in killing bacteria more quickly. However, raising the temperature to 37 °C did not help dissolve tissues more effectively. Though we may think of raising the temperature of irrigants to kill bacteria more effectively, we should not raise the temperature more than a few degrees above body temperature as this may have harmful effects on the cells of the periodontal ligament (48).
Different devices for warming the NaOCl syringes (Figure 7.7) have been released into the market, but these devices are not capable of maintaining any raise of temperature. The best way of heating up the NaOCl is to do it in situ with an ultrasonic device.
Figure 7.7 Heating devices for NaOCl.
(Courtesy of Vista Dental Products.)
A recent study evaluated and compared the effects of concentration, temperature, and agitation on the tissue-dissolving ability of sodium hypochlorite (48). The results showed that weight loss (dissolution) of the tissue increased almost linearly with the concentration of sodium hypochlorite. Higher temperatures and agitation considerably enhanced the efficacy of sodium hypochlorite. The effect of agitation on tissue dissolution was greater than that of temperature; continuous agitation resulted in the fastest tissue dissolution.
Agitation
Moorer and Wesselink (14) found that the impact of mechanical agitation of hypochlorite solutions on tissue dissolution was very important and they emphasized the great impact of violent fluid flow and shearing forces caused by ultrasound on the ability of hypochlorite to dissolve tissue. Stojicic et al. (45) found that refreshing the hypochlorite solution at the site of dissolution by agitation, preferably continuous, also resulted in a marked increase of hypochlorite effect. Fabiani (49) also demonstrated that the use of ultrasonic agitation increased the effectiveness of 5% NaOCl in the apical third of the canal wall. Finally, passive ultrasonic irrigation with a nickel–titanium tip produced superior tissue-dissolving effects as compared to sonic irrigant activation (50).
Influence of NaOCl on bond strength
NaOCl irrigation leads to decreased bond strength between dentin and resin cements and may require a reversal agent because of its ability to affect the polymerization of the resin sealer (51).
Chlorhexidine (CHX)
History
CHX (Figure 7.8) was developed more than 50 years ago at Imperial Chemical Industries in England, and was first marketed in the United Kingdom in 1953 as an antiseptic cream (52). Since 1957, it has been used for general disinfection purposes and for the treatment of skin, eye, and throat infections in both humans and animals (52, 53).

Figure 7.8 CHX dark container for storage.
Molecular structure
CHX belongs to the polybiguanide antibacterial family, consisting of two symmetric 4-chlorophenyl rings and two biguanide groups connected by a central hexamethylene chain. CHX is a strong basic molecule and is stable as a salt. CHX digluconate salt is easily soluble in water (Figure 7.9) (54).
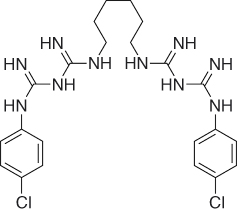
Figure 7.9 CHX molecule.
Mode of action
CHX is a wide-spectrum antimicrobial agent, active against gram-positive and gram-negative bacteria, and yeasts (55). Owing to its cationic nature, CHX is capable of electrostatically binding to the negatively charged surfaces of bacteria (56), damaging the outer layers of the cell wall and rendering it permeable (57–59).
Depending on its concentration, CHX can have both bacteriostatic and bactericidal effects. At high concentration, CHX acts as a detergent, and by damaging the cell membrane it causes precipitation of the cytoplasm and thereby exerts a bactericidal effect. At low sublethal concentrations, CHX is bacteriostatic, causing low molecular weight substances, that is, potassium and phosphorus, to leak out without the cell being irreversibly damaged. It also can affect bacterial metabolism in several other ways such as abolishing the activity of the phosphotransferase system (PTS) sugar transport system and inhibiting acid production in some bacteria (60).
Substantivity
Owing to the cationic nature of its molecule, CHX can be absorbed by anionic substrates such as the oral mucosa (61, 62). CHX has the ability to bind to proteins such as albumin, which is present in serum or saliva, pellicle found on the tooth surface, salivary glycoproteins, and mucous membranes (63, 64). This reaction is reversible (65). CHX can also be adsorbed onto hydroxyapatite and teeth. Studies have shown that the uptake of CHX onto teeth is also reversible. This reversible reaction of uptake and release of CHX leads to substantive antimicrobial activity and is referred to as substantivity (66). This effect depends on the concentration of CHX. At low concentrations of 0.005–0.01%, a stable monolayer of CHX is adsorbed and formed on the tooth surface, which might change the physical and chemical properties of the surface and may prevent or reduce bacterial colonization. At higher concentrations (>0.02%), a multilayer of CHX is formed on the surface providing a reservoir of CHX, which can rapidly release the excess into the environment as the concentration of the CHX in the surrounding environment decreases (67).
The antibacterial substantivity of three concentrations of CHX solution (4%, 2%, and 0.2%) after 5 min of application has been evaluated. Results revealed a direct relationship between the concentration of CHX and its substantivity (68). On the contrary, Lin et al. (69) attributed the substantivity of CHX to its ability to adsorb on to the dentin during the first hour. They stated that it is only after the saturation point is reached after the first hour that the antimicrobial capability of CHX increases with time. Furthermore, Komorowski et al. (70) revealed that 5 min application of CHX did not induce substantivity and that the dentin should be treated with CHX for 7 days. Taken together, it seems that residual antimicrobial activity of CHX in the root canal system remains for up to 12 weeks (68).
Cytotoxicity
In the medical field, CHX is normally used at concentrations between 0.12% and 2.0%. According to Löe (71), at these concentrations, CHX has a low level of tissue toxicity, both locally and systemically (71). Another report stated that when 2% CHX was used as a subgingival irrigant, no apparent toxicity was noted on gingival tissues (71, 72). Moreover, CHX rinse was reported to promote the healing of periodontal wounds (73). On the basis of these reports, Jeansonne and White (74) assumed that the apical tissues would be as tolerant to CHX as gingival tissues. In two studies it was found that when CHX and NaOCl were injected into the subcutaneous tissues of guinea pigs and rats, an inflammatory reaction was developed; however, the toxic reaction to CHX was less than that to NaOCl (75, 76). Furthermore, when CHX was applied as a rinse in the extraction sites of the third molars on the day of surgery and several days after, it was reported to reduce the incidence of alveolar osteitis (77). In addition, there are only a few allergic and anaphylactic reactions reported to CHX (78, 79).
Conversely, some studies have reported unfavorable effects of CHX on the tissues. Hidalgo (80) demonstrated that CHX is cytotoxic to some lines of cultured human skin fibroblasts. Recently, the behavior of osteoblastic human alveolar bone cells in the presence of CHX and povidone–iodine (PI) has been investigated. It has been reported that CHX has a higher cytotoxicity profile than PI (81). Faria et al. (82) also demonstrated that CHX injected into the hind paws of mice could induce severe toxic reactions. In addition, they reported that CHX induced apoptosis at lower concentrations and necrosis at higher concentrations when added to cultured L929 fibroblast cells.
Another interesting observation has been reported recently when CHX is in contact with other agents such as NaOCl. The by-product of the reaction of CHX with NaOCl is the formation of toxic breakdown products such as para-chloroaniline (PCA) that may have a negative impact on tissues (83). The toxicity level of CHX on apical tissues when applied in the root canals needs to be investigated further.
Chlorhexidine application in dentistry
CHX has several applications in dentistry. It has been used for the prevention of dental caries, plaque formation, and gingivitis, especially in the elderly and senile patients, as well as those with conditions such as cerebral palsy and patients with immune-compromising diseases. It has also been recommended for the prevention of alveolar osteitis after the extraction of third molars. Another application of CHX is in the treatment and management of periodontal diseases, as well as in the reduction of the incidence, severity, and duration of aphthous ulceration. In addition, it has been advocated as a denture disinfectant in patients susceptible to oral candidiasis. CHX can be prepared in the form of mouth rinses, gels, varnishes, and controlled-release devices (83).
Chlorhexidine application in endodontics
In endodontics, CHX has been studied as an irrigant and intracanal medication, both in-vivo (84–87) and in-vitro (88–91). In-vitro, CHX has at least as good, or even better, antimicrobial efficacy than Ca(OH)2 (92). Notably, 2% CHX is very effective in eliminating a biofilm of E. faecalis (85). In-vivo, it inhibits experimentally induced inflammatory external root resorption when applied for 4 weeks (84). In infected root canals, it reduces bacteria as effectively as Ca(OH)2 when applied for 1 week (93). Unlike Ca(OH)2, CHX has substantive antimicrobial activity that, if imparted onto the root dentin, has the potential to prevent bacterial colonization of root canal walls for prolonged periods of time (70, 74). This effect depends on the concentration of CHX, not on its mode of application, which may be used either as liquid, gel, or a controlled-release device (89).
Chlorhexidine as an endodontic irrigant
CHX in liquid and gel form has been recommended as an irrigant solution, and its different properties have been tested in several studies, both in-vitro (9) and in-vivo (93–100).
Many investigations have been conducted to study the antibacterial effectiveness of CHX in different concentrations. It has been demonstrated that 2% CHX as an irrigant has a better antibacterial efficacy than 0.12% CHX in-vitro. Thus, it is concluded that the antibacterial efficacy of CHX depends on its concentration level (90). Because NaOCl is still the most commonly used irrigant, the antibacterial efficacy of CHX is tested against that of NaOCl. The results from these studies are not conclusive, but in general no significant difference between these two solutions has been reported. It is possible, however, that culture methods are not sensitive enough to detect differences in the antibacterial effectiveness of various antibacterial agents; that is, the methods may not be suitable to quantitate the killing of biofilm bacteria. Unlike NaOCl, CHX lacks the tissue-dissolving property. Therefore, NaOCl is still considered to be the primary irrigation solution used in endodontics.
The cleanliness of root canals using CHX in gel and liquid forms was evaluated with SEM in two separate experiments. In an in-vitro study, the canals treated with 2% CHX gel were cleaner than those treated with 2% CHX liquid or 5.25% NaOCl, and it was suggested that the mechanical action of the gel might have facilitated the cleansing of the canals. Another in-vitro study showed that the 2% CHX liquid was inferior to 2.5% NaOCl in cleaning the canals (101). However, in-vitro studies may not properly reflect the actual in-vivo situations, which are more clinically relevant.
The antibacterial effectiveness of CHX in the reduction of bacteria in infected root canals in-vivo has been investigated in several studies. Ringel et al. (102) reported that 2.5% sodium hypochlorite was significantly more effective than 0.2% CHX when the infected root canals were irrigated for 30 min by either of the solutions.
In a controlled and randomized clinical trial, the efficacy of 2% CHX liquid was tested against saline using a culture technique. All the teeth were initially instrumented and irrigated using 1% sodium hypochlorite. Then, either 2% CHX liquid or saline was applied as a final rinse. The authors reported a fu/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


