Healing of Apical Lesions: How Do They Heal, Why Does the Healing Take So Long, and Why Do Some Lesions Fail to Heal?
Zvi Metzger and Anda Kfir
Department of Endodontology, Tel Aviv University, Tel Aviv, Israel
Introduction
Apical lesions are radiolucent lesions that appear in the bone surrounding portals of exit from infected root canal systems. Because most lesions of this type occur in the apical area, and for the convenience of the reader, the term apical lesion is used in this chapter. Many but not all apical lesions will heal in response to adequate debridement, disinfection, and obturation of the root canal. Such healing may be a prolonged process, and some lesions will fail to heal. To understand why the healing process is often prolonged and why some lesions fail to heal, the nature of these lesions and the processes leading to their development must be understood. Because the healing of apical lesions occurs via the regrowth of bone into the area, an understanding of the osteogenic signals that lead to and control the apposition of new bone is also important.
Some apical lesions fail to respond to intracanal endodontic treatment. However, many of them will heal after subsequent apical surgery has been applied. The reasons for such failures are discussed in an attempt to understand why apical surgery does lead to healing in many cases that originally failed to heal. This discussion is extended beyond the simple concept of a retrograde approach to the root canal system, to include the eradication of extraradicular infection and the removal of cystic formations and other factors, the elimination of which may represent additional factors contributing to the success of apical surgical intervention.
What is the apical lesion?
A protective host response with a price tag
Apical lesions represent a protective activity of the host response that is successful most of the time. Nevertheless, this protection has a price tag, which is destruction of the surrounding apical bone. Bone destruction is one of the primary indicative signs of an apical lesion. The gradual disappearance of the bone defect that was caused by this destructive response is commonly used as a major clinical sign and tool to monitor the healing of these lesions (1–6).
The protective response
The apical lesion represents a successful attempt of the host to prevent highly pathogenic bacteria present in the infected root canal from spreading into the adjacent bone and to other more remote places in the body (Figure 15.1). Some bacterial species that are often found in the apical part of the infected root canals, such as Porphyromonas gingivalis, Fusobacterium nucleatum, and Prevotella nigrescens, are extremely pathogenic (7, 8). Some strains of P. gingivalis can kill a mouse into which they are injected within 24 h (8), while other strains may cause severe spreading abscesses at the site of injection (7, 8). Furthermore, cooperation
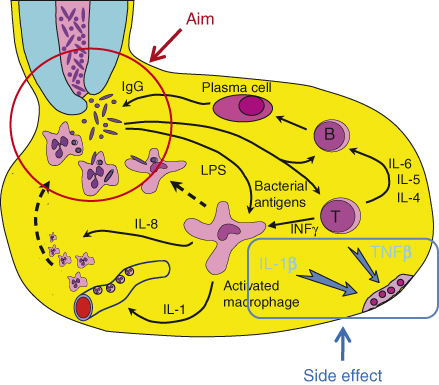
Figure 15.1 Host response in apical granuloma. The aim of the host response is to kill bacteria emerging from the infected root canal. To serve this aim, specific IgGs are required. These IgGs may be produced locally by activation of B-lymphocytes, which then become plasma cells secreting the IgG. This process requires prior local activation of antigen-specific T-lymphocytes. Activated lymphocytes produce an array of cytokines, some of which are required for B-lymphocyte activation and maturation to plasma cells. Gamma-interferon is another T-lymphocyte-derived cytokine that activates local macrophages and causes them to produce IL-1, which in turn induces the expression of attachment molecules on local endothelial cells. This causes PMNs to attach to the local endothelium, making them available for recruitment by chemotaxis to the site where bacteria emerge. Two of the cytokines produced by locally activated lymphocytes and macrophages, TNFβ and IL-1β, are the primary signals that induce local osteoclastic bone resorption. Such bone resorption may be viewed as a destructive side effect of the local activity of the host response.
between strains of F. nucleatum and P. gingivalis in the form of coaggregation (9) may make these strains 1000 times more pathogenic than each one alone (10). Osteomyelitis of the maxilla or the mandible is extremely rare. The protective mechanisms in the apical lesion are highly effective and contain the hazardous bacteria within the lesion in most cases. Occasional failures may occur, resulting in the development of an acute abscess.
The host response to bacteria is mediated by several processes: (i) the effective recruitment of polymorphonuclear granulocyte neutrophils (PMNs) to the site of bacterial penetration; (ii) effective opsonization with both specific immunoglobulin G (IgG) (11) and the complement component C3b; and (iii) effective phagocytosis of the bacteria, followed by intracellular killing by oxidative mechanisms. The host response in the apical lesion may be viewed as a complex mechanism for the recruitment of PMNs to the site at which the bacteria emerge from the root canal and for assisting the PMNs in effective phagocytosis of these bacteria (Figure 15.1) (12–14).
Prolonged exposure of the host to bacteria residing in the infected root canal is likely to result in the production of specific immunoglobulins against these bacteria. IgG specific to root canal bacteria have been found in human apical lesions (15–17). Although these specific IgGs may come from the systemic sensitization of the host, local production of such IgGs by plasma cells present in human apical lesions has also been reported by Baumgartner and Falkler (18) (Figure 15.1). Thus, bacteria emerging from the root canal are likely to encounter specific IgGs that attach to their surfaces. Such attachment will in turn activate the complement system, resulting in the generation of three signals: (i) the C3b elements of the complement system will attach to the surface of the bacteria and, together with the already attached IgG, will serve as effective opsonization mediators that permit subsequent phagocytosis by PMNs; (ii) the C3a and C5a complement components will cause the degranulation of local mast cells, which will release vasoactive amines; these released agents will cause the increased permeability of blood vessels in the area, in turn resulting in an increased supply of complement and specific IgG in the area; and (iii) C5a molecules will serve as a chemotactic signal for PMNs, directing them from the vicinity of the local blood vessels to the site of bacterial penetration.
PMNs normally circulate in the bloodstream and must be “told” where to exit the blood vessels so they can reach the invading bacteria. Interleukin-1 (IL-1), which is produced by activated macrophages in the apical lesion (19–21), serves as such a signal (Figure 15.1). When capillary endothelial cells are exposed to IL-1, they express attachment molecules such as ICAM-1 (inter cellular adhesion molecule-1) on their surfaces (22–25). PMNs in the blood bind to these attachment molecules and thus become concentrated and “marginated” in the area in which they are required. Guided by the concentration gradient of C5a molecules, the PMNs migrate into the tissue and move in the direction of the bacteria in a process known as directed chemotactic movement. It is important to note that PMNs are not resident cells of the apical tissue. Every PMN observed in a histological section of the tissue has been “captured” during the process of such chemotactic migration (13).
Once PMNs reach the bacteria, specific receptors for C3b and for the Fc portion of IgG allow the PMN to attach to opsonized bacteria that carry this dual signal on their surfaces. The PMN then internalize the bacteria through a process of phagocytosis, followed by the oxidative killing of the bacteria within the PMN.
Macrophage activation is essential for the local production of IL-1. Such activation is mediated by the cytokine gamma-interferon (γ-INF), which is produced by activated T-lymphocytes within the lesion (26–31) (Figure 15.1). The activation of T-lymphocytes is antigen-specific. Bacteria emerging from the root canal are phagocytized by antigen-presenting cells in the lesion, which process their specific antigens and present them to antigen-specific T-lymphocytes. This antigen presentation signal, together with IL-1, which is also produced by the antigen-presenting cells, causes the T-lymphocytes to become activated and produce many cytokines, one of which is γ INF, which is essential in turn for the activation of the macrophages. Other cytokines produced by the activated T-Lymphocytes such as IL-4, IL-5, and IL-6 are essential for the proliferation of antigen-specific B-lymphocytes and their maturation to plasma cells that will produce the antigen-specific IgG (Figure 15.1).
Thus, the apical lesion may be viewed as a complex mechanism that is designed to facilitate and support a single primary target: the phagocytosis and killing of bacteria by PMNs (13).
Development of apical lesions
The body’s response to the bacteria emerging from the apical foramen is initiated in the adjacent periodontal ligament in the form of apical periodontitis. This response, which is aimed at containing and killing the bacteria, also causes local damage to the host in the form of bone resorption (Figure 15.1). Among the cytokines that are produced by the cells of the apical inflammatory response, IL-1β and tumor necrosis factor β (TNF β) have the capacity to activate local osteoclastic bone resorption. The first (IL-1β) is produced mainly by activated macrophages, while the second (TNF β) is a product of activated T-lymphocytes.
IL-1β and TNF β are the primary causes of the local apical bone resorption (32). When lining cells of the bone are exposed to these cytokines, they express on their surfaces a signaling molecule, the receptor activator of nuclear factor kappa β-ligand (RANKL) (33–38). This ligand engages the RANK receptor, which is present on the surface of the neighboring preosteoclasts and osteoclasts, thus causing the maturation of preosteoclasts into mature osteoclasts and the activation of existing osteoclasts, which express ruffled borders and begin the bone resorbing actively (39–44).
The resulting local bone resorption is first radiographically expressed as a widening of the apical periodontal space; this space gradually increases, eventually resulting in a radiolucent lesion in the apical bone, that is, an apical lesion.
Apical bone resorption may thus be considered a side effect of the protective host response (Figure 15.1). The activation of an effective host response that is aimed at eliminating harmful bacteria results in the local production of cytokines that cause resorption of the surrounding bone (12, 13).
Granuloma versus abscess
Lesions of apical periodontitis usually contain inflammatory tissue in which lymphocytes, macrophages, and the resident cells of the periodontium, fibroblasts, are the dominant cells (Figure 15.2) (12, 45–49). Inflammatory lesions with such constituents are termed granulomas. Varying numbers of PMNs (Figure 15.3) may be found in granulomatous lesions, mainly adjacent to the apical foramen. It should be kept in mind that while lymphocytes, macrophages, and fibroblasts are long-lived cells, PMNs are not.
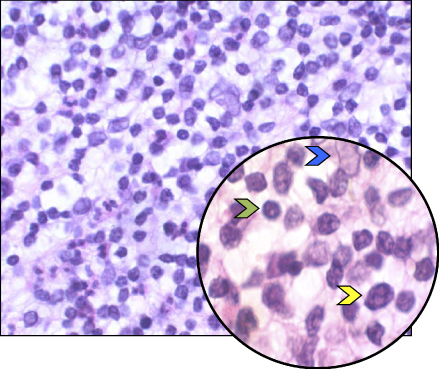
Figure 15.2 Apical granuloma. Histological section of an apical granuloma. Green arrow: Lymphocyte. Yellow arrow: Macrophage. Blue arrow: Fibroblast.
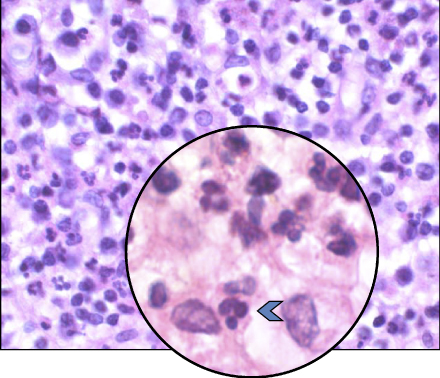
Figure 15.3 PMNs in an apical granuloma. Histological section of an apical granuloma. Blue arrow: PMN.
Any PMN observed in a lesion of apical periodontitis is recruited from the bloodstream and is in the process of migrating to the site of bacterial penetration, guided by chemotactic signals that consist of C5a components of the complement system. Every such PMN will die a preprogrammed death within 24–48 h of leaving the bloodstream (50).
When PMNs die, the proteolytic enzymes contained within them are released. These enzymes attack and damage or destroy the collagen and hyaluronic acid components of the connective tissue matrix (51). As long as the number of PMNs reaching the apical site per day is limited, the damage to the tissue is effectively repaired by local macrophages. The macrophages phagocytize the damaged tissue components and the remains of dead bacteria released from the PMN, thus performing a “cleanup” function. The macrophages also signal the fibroblasts to form new collagen and hyaluronic acid, resulting in the repair of the damage caused by the enzymes released from the dead PMNs. Conversely, if excessive numbers of PMNs reach the site, massive proteolysis of tissue components may occur, which is beyond the “cleanup” and repair capacity of the macrophages in the area. Under such conditions, local liquefaction of the tissue occurs and pus forms (13).
The appearance of an abscess within a granuloma may be viewed as the result of a disturbance of the equilibrium between the damage caused by the released PMN enzymes and the cleanup and repair capacity of the macrophages (12, 13). Because the number of macrophages in the lesion is more or less stable, any event that results in massive recruitment of PMNs is likely to induce an abscess within the granuloma. Such an event may be transient in nature, such as accidental pushing of bacteria into the apical lesion by an endodontic file. In such a case, the abscess will eventually subside, and with time, the macrophages will remove the damaged tissue and induce repair by fibroblasts. In other cases, there may be a persistent and continuous influx of large numbers of PMNs. This can occur when bacteria that have phagocytosis-evading mechanisms (7, 52–55) or bacteria that collaborate with other bacteria to form aggregates or biofilms (10, 56–59) are present in the root canal (see below). In such cases, the continuous influx of PMNs will result in continuous and persistent formation of pus and in the development of a chronic abscess with a permanently draining sinus tract.
Apical granulomas often contain epithelial elements originating from the rests of Malassez (60) (Figure 15.4). This epithelium may proliferate in response to root canal infection (20, 21, 61–65) or overinstrumentation and filling beyond the apex of the tooth (66). Such proliferation may eventually lead to development into cystic formations (see below).
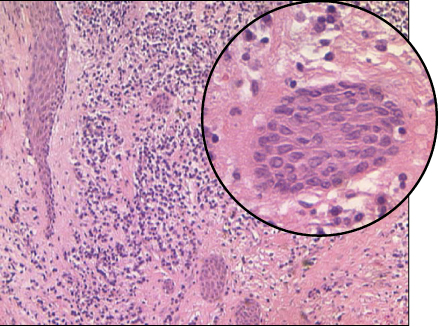
Figure 15.4 Epithelial proliferation in an apical granuloma. Strands of epithelium in an apical granuloma. Some strands were cut longitudinally; others were cut diagonally, giving the impression of isolated isles.
The lumen of such cystic formations may or may not be infected, and they may be either bay (pseudo) cysts or true cysts (62, 67) (see below).
How does an apical lesion heal?
Basic concepts of osteogenesis
Bone formation in the apical area, as well as elsewhere in the body, is dependent on the activity of osteoblasts, the bone-forming cells. Osteoblasts originate as mesenchymal stem cells in the bone marrow (Figure 15.5). Under the influence of bone morphogenic proteins (BMPs), these stem cells are induced to differentiate and give rise to spindle-shaped osteoprogenitor cells. Growth factors such as transforming growth factor β (TGFβ), fibroblast-derived growth factor (FGF), BMPs, platelet-derived growth factor (PDGF), and colony-stimulating factor (CSF) can induce and/or increase the proliferation of and are chemotactic to osteoprogenitor cells (68). Osteoprogenitor cells may accumulate at a future site of bone formation by local proliferation, by the chemotactic attraction of osteoprogenitor cells from adjacent sites, or both processes (68) (Figure 15.5).
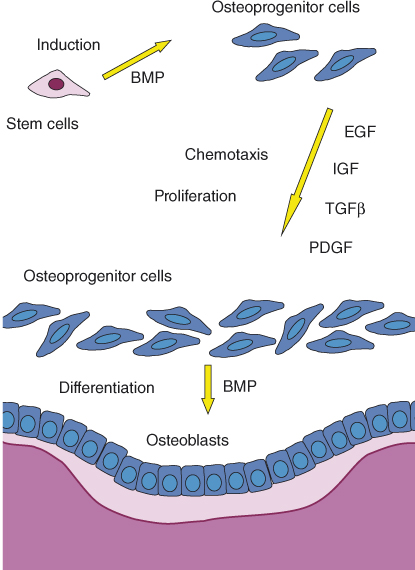
Figure 15.5 Apposition of new bone. Osteoprogenitor cells are required for the formation of new osteoblasts and new bone. Osteoprogenitor cells originate as bone marrow mesenchymal stem cells that are induced by BMPs to become osteoprogenitor cells. Certain growth factors, including epidermal growth factor (EGF), insulin-like growth factor (IGF), TGFβ, and PDGF, are chemotactic to the osteoprogenitor cells and cause them to proliferate. Consequently, spindle-shaped osteoprogenitor cells accumulate next to the future site of bone apposition. BMPs cause the final differentiation of the osteoprogenitor cells into cuboidal, metabolically active osteoblasts that line the bone surface and produce osteoid (shown in pink) that will later mineralize and turn into bone (shown in purple).
BMPs induce the final differentiation of the osteoprogenitor cells into cuboidal, metabolically active osteoblasts that line the bone surface and begin the process of bone apposition (68). The osteoblasts secrete collagen and BMPs as well as several growth factors and form the osteoid that will eventually mineralize and form bone. When osteoid apposition is completed, the osteoblasts differentiate into flat lining cells that cover the new bone.
Sources of osteogenic factors in the apical lesion
The tissues of the apical lesion and the surrounding bone are rich sources of the cells and signals required for the generation of active osteoblasts. Osteoprogenitor cells are found in the bone marrow, from which they can be attracted by chemotactic signals (68). Osteoprogenitor cells were also demonstrated to be present within the apical granulomas (69). Macrophages, which are abundant in apical granulomas, are a rich source of signals such as TGFβ, FGF, PDGF, and CSF, which are required for the recruitment and proliferation of osteoprogenitor cells. Platelets in blood clots formed after apical surgery are a particularly rich source of PDGF. BMPs, which are required for the final maturation of osteoblasts, are released locally from the resorbing bone matrix and are produced by neighboring osteoblasts (68). Thus, the apical granuloma and its surrounding bone are rich sources of components that together represent substantial osteogenic potential.
The remodeling process
Bone apposition is not a continuous process. The initially formed bone will eventually be resorbed and replaced by new bone formations in cycles of a process known as bone remodeling. Evidence for such resorption–apposition cycles can later be seen in the bone in the form of apposition lines (Figure 15.6a). Thus, one may envision the process of bone healing in the apical lesion as involving repeated cycles of apposition and remodeling. Such process is schematically illustrated in Figure 15.6b,c.
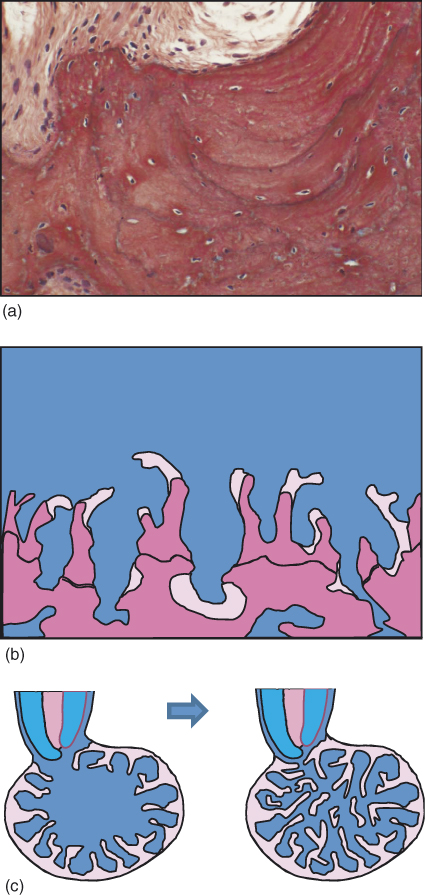
Figure 15.6 Bone remodeling. The process of bone formation occurs in cycles of formation and resorption that determine the final bone structure. (a) Reversal lines in bone representing cycles of resorption and apposition within the bone. (Reproduced with permission of Prof. Miron Weinreb, Tel Aviv University.) (b) Schematic presentation of the formation of bone trabeculae. Pink: Recently formed bone. Purple: Older calcified bone, with resting lines representing older bone resorption and apposition cycles. (Adopted from Aanan et al. (70). Reproduced with permission of Lippincott Williams & Wilkins.) (c) Schematic representation of bone formation in an apical lesion.
Healing of the apical lesion
The persistence of an apical lesion and its size are likely to be an expression of the local balance between the osteoclastic activity within the lesion and the osteogenic potential that surrounds it (Figure 15.7). The bacteria emerging from the infected root canal provide a stimulus for activation of T-lymphocytes and macrophages thus maintaining the osteoclastic signals in the lesion. When these bacteria have been eliminated by root canal treatment and the canal has been properly sealed, this stimulus ceases to exist, and with time, the response to the bacteria subsides. The osteoclastic activity, initiated by IL-1β and TNFβ, will then diminish, and the surrounding osteogenic potential will take over (Figure 15.7). The gradual apposition of new bone, followed by its remodeling and further cycles of apposition, will eventually result in the healing of the bone defect that was initially caused by the response to the bacteria, and the apical lesion will heal (Figure 15.6c).
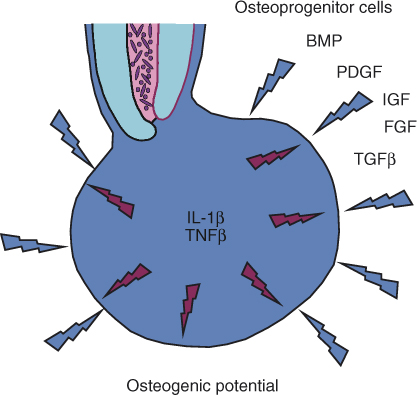
Figure 15.7 Osteoclastic versus osteogenic potential in an apical granuloma. The cytokines TNFβ and IL-1β, which are produced by activated T-lymphocytes and activated macrophages in the lesion, serve as the primary main signals that induce osteoclastic bone resorption in an apical granuloma. The surrounding bone is a rich source of osteoprogenitor cells that, together with locally produced EGF, IGF, TGFβ, PDGF, and BMPs, provide osteoblastic potential. Once bacteria are eliminated from the root canal, gradual yet slow reduction of the local production of TNFβ and IL-1β occurs, and the osteogenic potential begins to dominate, causing healing of the lesion by new bone apposition.
How long does it take the lesion to heal?
Several large-scale follow-up studies indicate that 74–85% of apical lesions heal within 48 months (1, 2, 4–6).
Ørstavik’s study (1) found that 85% of such lesions healed within 48 months. Of the lesions that eventually healed, only 50% were healed or in a process of healing at 6 months after treatment, namely 42.5% of the total number of lesions that were studied (50% of 85%). At 12 months, 88% of the lesions that eventually healed were healed or in the process of healing, namely 75% of the total number of lesions (88% of 85%) (1).
Thus, healing of an apical lesion is a rather prolonged process. If a cavity of similar size is surgically formed in the bone, healing occurs much more rapidly (71).
Why does healing often take so long?
Macrophage activation
Activated macrophages and activated lymphocytes are the primary sources of IL-1β and TNFβ cytokines, which represent the main osteoclast-stimulating activity in the apical lesion (32) (Figure 15.1). Animal studies have shown that macrophage activation that is induced, for example, by the subcutaneous injection of streptococcal cell walls may persist for a very long period of time (72).
A potential explanation for the extended time required for apical lesions to heal may be the persistence of an activated state of macrophages and lymphocytes within the lesion. Such an activated state may outlive its biological purpose as a pivotal element of the host’s defensive response in the area of the lesion (12, 13). As long as such activation persists and these cytokines are produced, the osteoclastic potential of the lesion persists, keeping the vast surrounding osteogenic potential at bay. When the osteoclastic activity subsides, the lesion finally heals (12, 13).
Potential pharmacological intervention sites
A better understanding of the processes that are involved in the production and effects of the bone-resorbing cytokines may in the future permit pharmacological intervention in the balance between osteoclastic and osteogenic activities in the apical lesion and make it possible to favorably affect the kinetics of the healing process. Several potential targets for such pharmacological intervention are the local production and release of cytokines that induce osteoclastic activity (27, 73–76), the receptors for these cytokines on local target cells (77) and the bone-resorbing activity of the osteoclasts themselves (12, 13).
The effect of apical debridement
Another approach to enhancing the kinetics of the healing of apical lesions is the mechanical removal of the granuloma. A study by Kvist and Reit (71) compared the healing of apical lesions after retreatment and after apical surgery. The apical surgery used in that study did not include retrograde filling. Although healing after 48 months was similar in the two groups, healing after 12 months was substantially greater in the group in which the apical lesions were surgically removed (71).
A more recent study applied a microinvasive method to remove the bulk of the tissues from apical lesions with no open-flap surgery (78, 79) (see below). When the apical tissue was removed and allowed to be replaced by a blood clot that, in turn, developed into fresh, “uncommitted” granulation tissue, the healing kinetics were substantially enhanced (see below).
Taken together, these two studies support the idea that residual activated cells of the apical tissue remaining in the bony crypt of the lesion after the elimination of the bacteria from the root canal are the likely cause of the extremely long time that bony defects of this type sometimes require to heal (13).
Why do some lesions fail to heal?
Residual infection within the root canal system
Residual infection in the root canal system is commonly perceived as the reason for the failure of apical lesions to heal. It is indeed the most common reason, but it is not the only one, as discussed below.
The root canal system often includes components that are inaccessible to intracanal debridement and disinfection. Lateral canals, delta-like ramifications of the canal, and fin-like recesses of the main canal are among such inaccessible components. The ramifications of the canal are more common in the apical part of the canal; cutting off the tip of the root/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


