Chapter 10
Apical Negative Pressure Irrigation (ANP)
Nestor Cohenca
Department of Endodontics and Pediatric Dentistry, University of Washington, Seattle, WA, USA
Cesar de Gregorio and Avina Paranjpe
Department of Endodontics, University of Washington, Seattle, WA, USA
Introduction
It has been well established for the past several years that the etiology for endodontic disease is microbial pathogens. Toxic metabolites and by-products released from microorganisms within the canal diffuse into apical tissues and elicit inflammatory responses and bone resorption (1, 2). The host immune response plays an important role at this time by preventing the infection from spreading into the various parts of the body. However, because of the lack of blood supply in a necrotic tooth, the host response cannot affect the bacteria that are present in the root canal system. Hence it is necessary to mechanically and chemically remove these microorganisms during root canal treatment procedures. Irrigation along with the instrumentation of the canal can help achieve the goal of disinfection of the root canals. Irrigation supports mechanical instrumentation along with killing and removing any residual microbes left back in the canal. Currently, there are numerous irrigation solutions and irrigation techniques available and many of them have showed improvement even in the most complex canal systems. This chapter focuses on the apical negative pressure (ANP) irrigation system’s unique abilities, advantages, and benefits, one of which is exemplified in Figure 10.1.
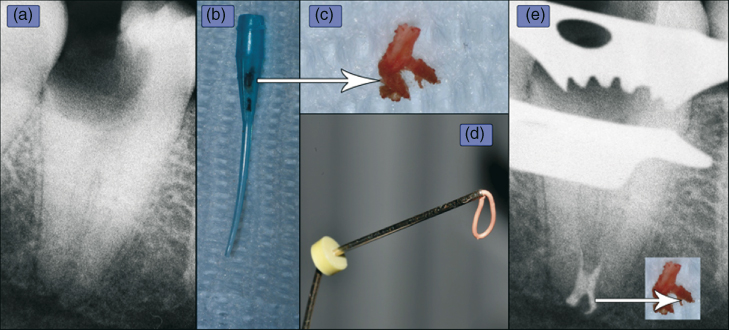
Figure 10.1 (a) An obviously infected root canal space demonstrating apical rarefaction and condensing osteitis. After instrumentation, the (b) macrocannula component of the EndoVac (ANP) system aspirated unexpected material (dark matter in cannula) that was in fact the (c) terminal portion (arrow) of the still vital, yet infected pulp tissue. Notice the lateral portion facing to the right. Injectable gutta-percha (d) was the method of obturation. (e) The lateral canal was obviously obturated and matches the shape of the recovered pulp tissue.
(Case courtesy: Dr. Filippo Santarcangelo.)
Positive pressure (PP) irrigation is still widely used by many clinicians. However, a review of the literature shows that PP irrigation systems have their limitations—inadequate debridement and disinfection in the apical area. Previous studies have demonstrated that PP irrigation had virtually no effect on the orifice of the needle (3). Furthermore, some more recent studies have demonstrated the effectiveness of other irrigation systems over the traditional PP systems (4–6). In addition to debridement and disinfection, safety is another concern with PP irrigation. Sodium hypochlorite (NaOCl) accidents have been described in the literature previously with some being more severe than others (7, 8). The main cause for this seems to be attributed to the amount of pressure created during PP irrigation in comparison to the capillary blood pressure (9, 10). More recent studies have also demonstrated the dangers of PP irrigation in relation to central venous pressure (11, 12).
ANP overview
Whatever irrigation procedure is used during a root canal treatment, it must be performed bearing in mind that effectiveness cannot supersede the safety of the patients. The main problem with PP and NaOCl accidents is the randomness of its nature. This unpredictability seems to be related to the apical status (13) and anatomical factors (14). Clinically, we cannot immediately diagnose when a significant amount of irrigant has been extruded to the apical tissues, and it is virtually impossible to predict these undesirable NaOCl accidents (15). Another drawback of PP irrigation is the apical stagnation plane (16, 17). This barrier allows for gas entrapment, produced by the decomposition of organic tissue. This physical phenomenon is called vapor lock making it difficult to adequately debride the canal’s apical termination (18, 19). In order to overcome all these drawbacks, the ANP technique for irrigation was introduced and is discussed in detail in this chapter.
The effectiveness of an irrigating solution is dependent mainly on its adequate diffusion into the root canal system and its volume. The depth of needle placement conditions, the irrigant penetration, and delivering of large amounts of irrigants have been related to high pressures (20). This is one of the main reasons why traditional irrigation systems fail to completely debride the root canal especially because they are placed in a safety depth of 2–3 mm of the working length (WL) to avoid the hypochlorite accident (3). This is not the case with the ANP systems, which because of its philosophy, has the ability to deliver irrigants up to the WL and eliminating any risk of apical extrusion (21). In addition, studies have demonstrated the effectiveness of the ANP systems to deliver irrigants to the WL in comparison with traditional irrigation, which also relates to the apical vapor lock phenomenon (18, 22).
The most significant advantage is its safety considerations. Studies have compared other irrigation systems to ANP, specifically the EndoVac, currently marketed by SybronEndo (SybronEndo, Orange, CA). Desai and Himel (21) in an in-vitro study demonstrated that the ANP did not extrude any irrigant while the groups including the Max-i-Probe (Dentsply Rinn, Elgin, IL), Rinsendo (Dürr Dental GmbH & Co. KG, Bietigheim-Bissingen, Germany), and the continuous ultrasonic irrigation (CUI) needle extruded significant amounts of irrigant that could irritant and be toxic to the apical tissues. This extrusion of irrigant could lead to significant postoperative pain. This parameter was examined by Gondim et al. (23) who reported significant reduction in postoperative pain levels when patients were treated using ANP as compared to traditional needle irrigation.
Orientation: This and the next paragraphs are presented as a precursor to more detailed discussions in this chapter. First, as ANP systems can be safely taken to WL, microbiological studies have demonstrated a statistically significant difference between ANP and PP irrigation in-vitro. The ANP groups have shown no positive cultures compared with the PP groups, and no significant differences between types of preparations and apical sizes have been noted (24). A recent randomized clinical study involving patients with apical apical periodontitis in lower molars confirmed these results (25). However, an increase in the apical size preparation from #35 to #40 and an increase in taper from 0.02 to 0.04 did demonstrate an increased volume of irrigant being delivered to WL, which in turn could contribute to the microbiological success when using this system (26, 27). Yet, some conflicting studies have been published and the reason for their different findings are thoroughly explained. For example, Pawar et al. (28) demonstrated the importance of following the manufacturer’s instructions when that study failed to demonstrate differences in efficacy between PP needle irrigation and ANP, greater than or equal to 5% NaOCl was not used. Six years earlier Clegg et al. (29) proved that 1% would not prevent culture growth.
In addition to its antimicrobial efficacy, there are many studies that have demonstrated the effectiveness of the ANP system for tissue dissolution, debris removal, and smear-layer removal. The classic study by Nielsen and Craig Baumgartner (30) performed in extracted teeth demonstrated significantly less debris with the ANP group at 1 mm from WL in comparison with conventional needle irrigation. Other in-vitro studies done in different teeth types and using different methodologies such as scanning electron microscopy (SEM) have demonstrated and supported Nielsen results using different methods (6, 31–34).
Besides being used in teeth with closed apices and for routine root canal treatment procedures, ANP irrigation has also been studied in regenerative endodontic procedures. The use of the ANP irrigation in dogs during regenerative endodontic procedures has proved to be a promising disinfection protocol suggesting that the use of the triple antibiotic paste may not be necessary (35, 36).
Rational
For years, numerous in-vitro studies using an open canal system, where the apical tissues were not simulated, have shown good irrigation results using PP irrigation (37). However, in the clinical situation, the root canal system is closed because of apical tissues. Tay et al. (38) conclusively demonstrated the importance of using a closed system when evaluating irrigation results. Efficient debridement of the root canal system and isthmuses was recently tested using two irrigant agitation techniques in a closed system and confirmed that the correct simulation of a closed system is essential to re-create the fluid dynamics occurring during the endodontic irrigation (31). The challenge is to predictably create a direct contact between the chemical substance and the complexity of the canal system plus a continuous renewal of the irrigant, thus allowing disinfection and removing debris, all this with no or minimal apical extrusion. These objectives are difficult to achieve by any system. It is a unique physical and anatomical situation that must be understood in order to find a solution toward a better endodontic outcome.
We can categorize irrigation devices into two major groups: activation and delivery systems. Activation systems aim to improve the movement of the irrigant within a complex space. Delivery systems, such as ANP, were developed to overcome the physical facts existing into a semipermeable canal system, thus allowing full penetration of the irrigant with no risk of extrusion. This is a new philosophy and actually contradicts the needle irrigation we have been using for more than 50 years.
Fukumoto et al. (39, 40) published the first articles explaining the rationale, and, in 2007, the first commercial ANP system, the EndoVac system (SybronEndo, Orange, CA), was introduced. Recently, another ANP system, INP (ASI Medical, Englewood, CO), has been introduced using materials and methods vastly different from the EndoVac system and it lacks research to provide evidence based on its efficacy. Fukumoto’s specimens were placed in a container colored salt agar with 1% of a dye (Caries Detector, Kuraray Co., Ltd., Osaka, Japan) with the aim of analyzing the irrigant and subsequent extrusion. This log samples were analyzed by SEM to observe the cleanliness produced by NaOCl, and ethylenediaminetetraaceticacid (EDTA), in the apical 3 mm of PP and ANP irrigation. The groups irrigated with ANP demonstrated better cleanliness in the apical millimeter. Extrusion results demonstrated that ANP system was significantly safer than PP, extruding a minimum amount of irrigant during the entire process. Irrigant extrusion was the same for ANP groups while the cannula, that is, at two different depths, not for PP, where the greater proximity of the needle to WL (3 mm) caused a greater extrusion, corresponding to previous studies (10). The findings of the study confirmed the effectiveness of intracanal aspiration system in clearing the last few millimeters of the preparations, as well as greater security of this system compared to irrigation with PP.
Basically, the ANP system includes four major components: a multiport adapter (MPA), master delivery tip (MDT), macrocannulas, and microcannulas. The MDT was designed to deposit the solution at the access and pulp chamber and autoevacuate any excess. The macro- and microcannulas are aimed at aspirating the irrigant solution into the canal creating dynamic flow with constant renewal of the solution, thus decreasing the loss of effectiveness of our irrigants to contact organic and inorganic residues. This allows us to employ large volumes of irrigants without the danger of causing damage to the apical area.
The device and clinical technique
EndoVac (SybronEndo, Orange, CA) was the first ANP system available commercially (Figure 10.2). It has been thoroughly investigated since 2007 and, as mentioned above, it is based on four main components: the hand and finger pieces, the macro- and microcannula, the MPA, and the MDT.
- The multiport adapter (MPA): The MPA plugs directly into Hi-Vac and serves as a caddy for the EndoVac tubing, and other components are easily removed and reattached to the Hi-Vac system for maximum portability between operatories (Figure 10.3).
- The master delivery tip (MDT): The MDT plugs directly into the blue port of the MPA and provides a constant flow of the irrigant without the risk of overflow. The MDT is used during coronal flaring and after each instrument change to remove gross debris arising from instrumentation (Figure 10.4c).
- The macrocannula is an ISO 55 plastic cannula used to remove coarse debris inside the canal after all instrumentation is completed. In this step, the macrocannula and the MDT are used at the same time. It is helpful to have a dental assistant delivering the irrigant with the MDT while the clinician works the macrocannula up and down each canal (Figure 10.4. The macrocannula is designed for single use and should be discarded after each treatment.
- The microcannula is a 30-gauge needle (0.32 mm) with 12 laser-drilled, microscopic evacuation holes—each less than 100 µm in size—all of them placed within the last 0.7 mm of the needle (Figure 10.4b,d). Fluid is drawn to the apical termination through these holes, creating a vortex-like cleaning of the apical third. The microcannula is designed for single use and should be discarded after each treatment.
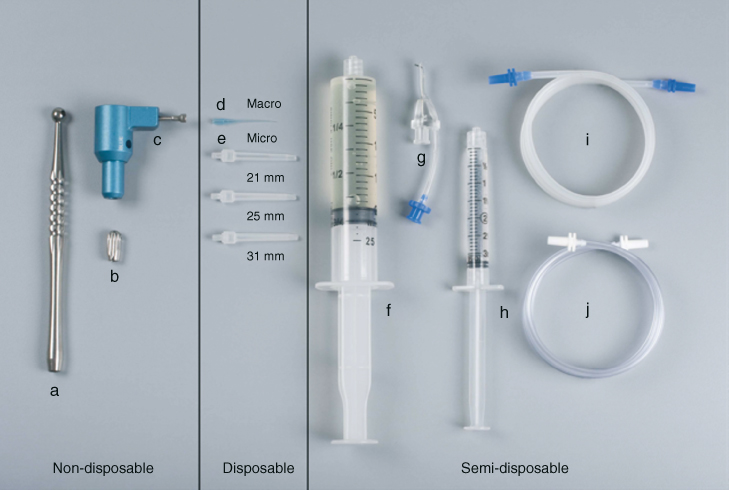
Figure 10.2 (a) Handpiece; (b) fingerpiece; (c) multiport adapter; (d) macrocannula; (e) microcannula (21, 25, 31 mm); (f) syringe 20 cc (for NaOCl); (g) master delivery tip (MDT); (h) syringe 3 cc (for EDTA); (i) MDT evacuation tubing (blue); and (j) handpiece/fingerpiece evacuation tubing (white).
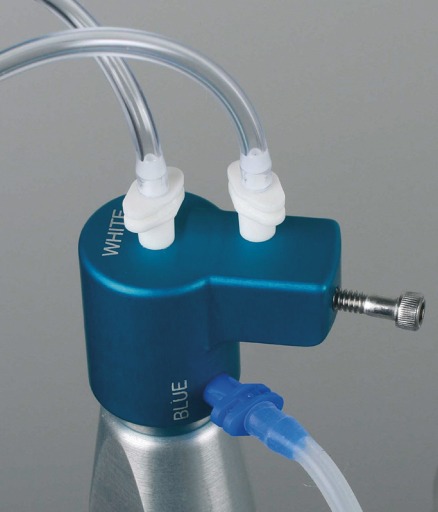
Figure 10.3 The multiport adapter.
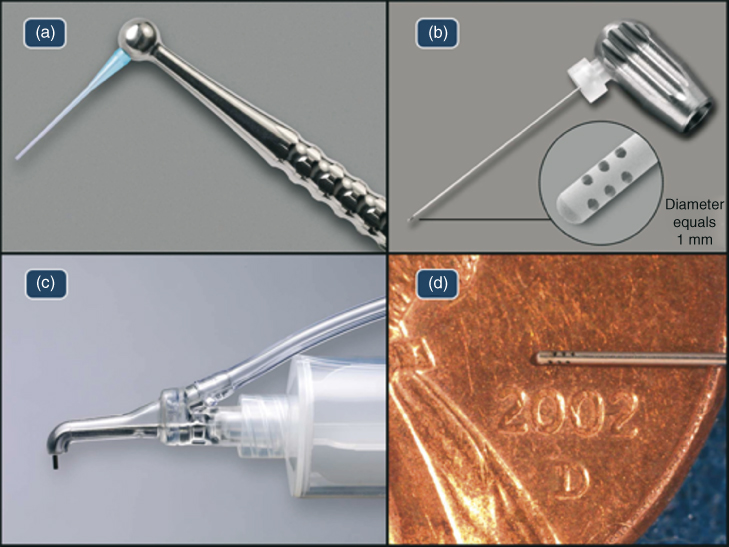
Figure 10.4 EndoVac components: (a) macrocannula; (b) microcannula demonstrating the evacuation holes in the apical 0.7 mm; (c) master delivery tip; and (d) microcannula.
Clinical technique
The EndoVac irrigation procedure uses two different cannulas of different materials size and configuration in each canal, already described as the macro- and microcannula. They are attached to Hi-Vac to create vacuum pressure, thus allowing them to pull the irrigants to their tips, thereby preventing apical extrusion while concurrently and omnidirectionally aspirating debris from the root canal system. The irrigants are delivered passively at the lip of the access opening via the MDT.
Canal instrumentation
During the entire instrumentation process, the MDT is used to supply 1 ml of 5–6% NaOCl before and after every instrument change. This replenishment process evacuates the instrumented debris drawn into the pulp chamber by the preparation instruments while concurrently providing fresh irrigant that will be worked down the canal by the next instrument. In a randomized clinical study, Cohenca et al. (25) determined that instrumentation strategies or types did not affect the efficacy of the EndoVac irrigation procedure. However, the minimum apical preparation size to accommodate the microcannula is 0.32 mm, being necessary an instrumentation with at least a #35/0.02 hand instrument to WL after smaller sizes of NiTi instrumentation is complete.
Macroevacuation
A macro is used to evacuate the gross debris from the root canal cavity after instrumentation. It is used for 30 s in each canal after instrumentation by rapidly moving it from a point where it stopped its apical progression to just below the pulpal floor as 5–6% NaOCl is passively delivered via the MDT at approximately 6–8 ml/min. Current flow is constantly monitored through the Macro’s transparent polypropylene wall to ensure blockage has not occurred. After 30 s of rapid irrigant exchange, the canal is left “Charged” with NaOCl by quickly withdrawing the macrocannula from the canal while continuing to deliver 5–6% NaOCl via the MDT. The canal is left undisturbed for 60 s (the “passive wait”) while the other canals are treated in the same method. In the case of a single-rooted tooth, the canal is left undisturbed for these 60 s.
Microevacuation
Micro irrigation begins immediately following the macro irrigation’s passive wait. Note—there is a definite learning curve to acquiring this technique—the most frequent mistake made by clinicians when learning this technique is the improper use of the Macrocannula as described above, resulting in unnecessary clogging of the Microcannula’s filtration holes. Once the micro is in place at full WL, the MDT delivers an uninterrupted flow of irrigant into the pulp chamber. During this irrigant application, the microcannula’s exhaust tube is observed to confirm irrigant flow.
Microcycles
Three irrigant “microcycles” (NaOCl, EDTA, and NaOCl) comprise microevacuation. The first microcycle clears the most apically positioned walls of debris and/or biofilm, as 6% NaOCl is delivered to the pulp chamber. After 6 s, the irrigant flow is stopped allowing the canal to be sucked dry (the “purge”) thereby evacuating the bubbles formed from hydrolysis; this is repeated four more times for a total of 30 s; then the microcannula is quickly withdrawn from the canal like the macrocannula before, leaving it charged for 60 s while the other canals are treated. After the NaOCl-charged passive wait, the second microirrigation cycle incorporating 17% EDTA was initiated to clear the smear layer. The microcannula is replaced at full WL and 17% EDTA is added for 10 s and the microcannula is again removed leaving the canal charged for an additional 60 s while the other canals are treated. As gas bubbles are not formed by the EDTA, purging is not necessary. Finally, with the gross debris and/or biofilm and smear layer removed from the canal walls, the tubules and the neighboring lateral and associated irregularities are treated via a second round of 5–6% NaOCl, as previously described, thus allowing NaOCl to diffuse into these areas. In the end, the canals are purged of all irrigant and one or two paper points are used to dry the canals. A representative clinical case is showed in Figure 10.5, demonstrating the different steps of ANP (Figure 10.5).
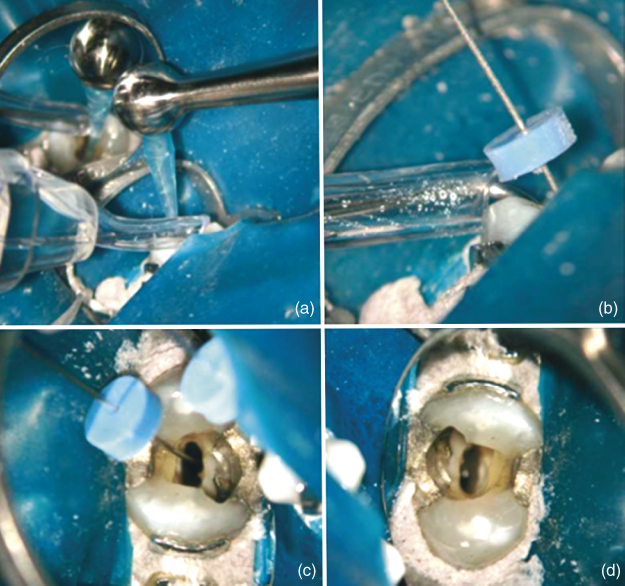
Figure 10.5 (a) Irrigation with macrocannula; (b) rubber stop of the microcannula placed at WL; (c) irrigation with the microcannula; and (d) canals after ANP.
Efficacy
The EndoVac’s effectiveness is based on its ability to create negative pressure ranging from approximately −30 to −260 mm Hg throughout the root canal system extending from the coronal access opening and terminating at the apical extent of the major diameter (41). This allows irrigants to be safely and effectively drawn in abundant quantities down and/or across the canal walls and/or through intracanal irregularities like isthmus areas and wall fins as they are added coronally and evacuated apically. Depending on the type of the irrigant used, organic and inorganic debris is hydrolyzed, chelated, and/or mechanically dislodged from the canal system and subsequently evacuated. Furthermore, this constant irrigant exchange extends beyond the fluid dynamics of delivery and evacuation by allowing the establishment of a diffusion gradient whereby hyperconcentrated solutions like 5% NaOCl diffuse into dead-end spaces (42).
Penetration
Regardless of the analytical method used, various studies evaluating the penetration of irrigants into the canal irregularities when delivered via ANP are in unanimous agreement. The first study that evaluated EndoVac’s ability to introduce the irrigant into main canal and canal irregularities was conducted by de Gregorio et al. in 2010 (43). This study used an in-vitro model consisting of cleared teeth embedded in wax to create a closed system, the irrigant solution used was a mix of 5.25% NaOCl and a dye in equal amounts. The irrigant was delivered short of the apical termination via PP and activated by either sonic or passive ultrasonic irrigation (PUI) or complemented with ANP irrigation (EndoVac). The importance of this model is that it could simultaneously measure the depth of the irrigant into the main canal and its penetration into the simulated noninstrumented lateral canals measuring 60 µm in diameter and placed at 2, 4, and 6 mm from the apical termination. Evaluation of these cleared teeth provided information about the penetration up to WL and the ability of the irrigant to be moved into the artificial lateral canals.
The root canals that were instrumented to an ISO #40 demonstrated complete penetration of the irrigant to the apical termination in all EndoVac samples; this was statistically significant compared to the other groups. This validated the microcannula’s ability to produce adequate ANP to resolve the vexing physical barrier problem, described in a “stagnation zone” (16) or the “vapor lock” (38). Regarding the penetration of artificial lateral canals, the EndoVac system, designed primarily to safely place abundant irrigant flow to the canal’s full length and not as an activation mechanism, was not as effective at filling the lateral canals as PUI. However, this limitation can be balanced by the diffusion effect described by Pashley et al. (44) even though this effect was not analyzed in cleared teeth. However, throughout the history, PUI has proved its efficacy cleaning noninstrumented areas and removing debris in relatively straight canals (45–48). However, van der Sluis et al. (49) stated that the resultant acoustic microstreaming depends inversely on the surface area of the file touching the root canal wall. Furthermore, Goode et al. in 2013 (41) demonstrated that ultrasonic activation could not effectively clean debris from a multiplaner canal; but the EndoVac produced significantly better debris removal than PP, manual dynamic, sonic, and ultrasonic activation.
Cohenca’s research team evaluated different irrigation systems in oval canals: EndoVac, PP, and self-adjusting file (SAF) system (50, 51). Results again confirmed the advantages of EndoVac, which delivered a full and constant irrigation at WL, showing significant differences compared to the other two systems. The conclusions of these two studies were confirmed by Spoorthy et al. (52) who tested the de Gregorio model but added a new experimental group: ANP + PUI. This new group of PUI + ANP showed better results in the most apical termination, demonstrating the synergistic effect of both techniques, and not affected by root canal curvature (53).
Munoz and Camacho-Cuadra (22) clinically evaluated the irrigant penetration in-vivo using a radiopaque contrast solution in mesial curved canals of mandibular molars. Because of the flexibility of microcannula, the effectiveness of EndoVac was confirmed, showing a statistically significant difference in the irrigation at full canal length in comparison with PP and similar results to PUI. However, it is important to realize the limitation of this study because Munoz and Camacho-Cuadra determined only the apical placement of the irrigant and not the critical volume of irrigant delivered or aspirated at the apical termination.
Debridement
Debridement is one of the basic three objectives of endodontic treatment. NaOCl’s solvent action dissolves and facilitates removing pulp remnants. As many studies have shown that chemomechanical preparation does not eliminate all the remnants, a proper delivery of NaOCl is needed to maximize its action (54, 55). With PP delivery, the debridement action of NaOCl is directly related to the flow rate of the irrigant and its apical pressure. This is emphasized at the end of this section. The higher intracanal pressure, the greater the cleaning effect is, but unfortunately the extrusion risk is higher; therefore, is important to rely on proper irrigation systems. Traditionally, to enhance debridement, increase of the diameter and taper apical preparation has been proposed (56, 57); however, the EndoVac’s safe delivery design enables abundant and safe irrigant delivery when the apical preparation is as small as a #35 ISO (21). Hockett et al. (24) concluded that ANP is crucial in the cleaning and disinfection of the canals than the use of bigger tapers.
Several recent studies have unanimously demonstrated positive proof regarding EndoVac’s debridement abilities within the root canal system. Susin et al. (31) selected 20 teeth with narrow isthmuses using micro-computed tomography (micro-CT); these samples were analyzed under light microscopy for the presence of debris in the canals of both, instrumented areas and isthmuses. For the analysis of this study, 10 slides were obtained and analyzed between 1 and 2.8 mm from WL. The groups included a manual dynamic activation (MDA) group with a fitted gutta-percha cone. Results did not show a significant difference between groups in the main canal; however, in the isthmuses areas, ANP was more effective removing organic debris (Figure 10.6).
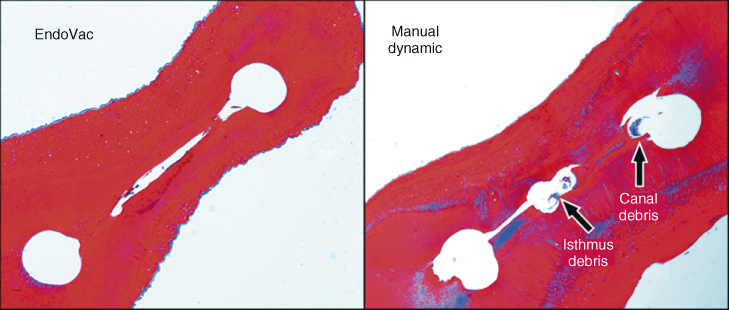
Figure 10.6 Both sections were taken at working length—1 mm. In the manual dynamic activation (MDA) group, greater isthmus debris was apparent and statistically significant at P = 0.001.
The results of this study are consistent with the outcome of the research by Siu and Baumgartner (5) who achieved better debridement in the very last millimeters when using ANP than PP group. According to the results of this study and that of Susin and Baumgartner, MDA showed a better cleaning than PP. PP and ANP showed similar results at coronal thirds; these can be explained as an effect at the level where the tip needle was placed, in concordance with the results obtained by Boutsioukis et al. (58, 59) using computational fluid dynamics (CFD), as higher stress was observed on dentinal walls, which could be influenced by the level, depth, and orientation of the tip needle.
In closed systems, debridement by PP is adversely affected by the presence of apical tissues (38). Parente et al. (32) showed that ANP is not affected by a closed system, while MDA had poor results in the same clinical conditions. Nielsen and Craig Baumgartner (30) used a very similar methodology and concluded that at the 1 mm level, significantly less debris was found in the EndoVac group (P ≤ 0.0347). Relevant data provided by this study are the volume of irrigants used and its influence on tissue dissolution. Within the same period of time, EndoVac allows the use of 42 ml of irrigant in comparison with 15 ml used in PP group.
It is interesting to note that Howard et al. (60) obtained different results than the previously discussed studies. They found no significant differences between ANP, PP, and CUI by PiezoFlow device (ProUltra, Dentsply, Tulsa, OK) in the mesial roots of the mandibular molars. However, a close examination of their materials and methods reveals a disturbing fact—the flow rate used during the CUI irrigation was set at 15 ml/min. Two years subsequent to this study, Khan et al. (11) tested the apical pressure generated by different irrigation techniques using nonbound needles placed at WL—1 mm up to a maximum delivery rate of 8 ml/min. At 8 ml/min, the CUI group produced 78 mm Hg apically directed pressure. Following this study, Zhu et al. (61), in a review manuscript, determined that a maximum flow rate of 3.5 ml/min for an unbound needle would produce 30 mm Hg apically directed pressure—the maximum limit for preventing an intravenous injection of an endodontic irrigant. Endodontic irrigation is a function of both safety and efficacy.
Smear layer
Effectiveness of ANP is based on a deeper penetration of the irrigant solutions, exposure time, and volume of irrigant. In the previous section, we reviewed the debridement efficacy of ANP, which has showed similar results removing smear layer. Currently, only a few studies have analyzed the effects of ANP on the smear layer. Saber Sel and Hashem (62) found that the activation of 17% EDTA either by MDA or ANP showed a statistically better smear-layer removal than with PP or PUI. However, at approximately the same time as Tay was validating the vapor lock theory (38) in a separate study, his team was investigating smear-layer removal in open and closed root canal systems using two different groups: MDA and ANP (32). The study concluded that the ability of manual dynamic agitation to remove smear layer and debris in a closed canal system was significantly less effective than in an open canal system and significantly less effective than the EndoVac (P < 0.001). Thus, they not only established the efficacy of the EndoVac to remove at the most apical depths of a closed canal system; they also reproved the vapor lock theory and called into question all former irrigation studies performed in open-ended samples.
In 2003, Torabinejad et al. (63) defined a scoring method for determining smear-layer removal. This method relies on analyzing the amount of smear layer covering the tubules. However, the tubules are scarce, inherently smaller, and look sclerotic in the walls in the very apical portion of the root canal (64). In 2006, Fukumoto et al. (40) devised a unique method to overcome this problem by amputating the apical 3 mm thereby assuring plentiful tubules at the apical extent of the modified canal. This study was the first reported use of ANP, and the SEM results demonstrated more effective removal of the smear layer than traditional irrigation. Gómez-Pérez (65) repeated Fukumoto’s study using the EndoVac system obtaining the same positive results (Figure 10.7).

Figure 10.7 SEM examination at WL—1 mm. (a) Saline control; (b) traditional irrigation; and (c) EndoVac irrigation.
(Dr. Arianna Gomez-Perez.)
Antimicrobial effect
The primary factor that determines the treatment outcome of well-treated and restored teeth is the biological status of the root canal system at the time of treatment. The success rate for noninfected root canal systems is about 92% (66, 67) while infected canal systems are generally only 80% successful (68). Since the early part of this century, the scientific community began to suspect the pathogenic role of biofilm within the root canal system as opposed to a solitary organism like Enterococci (69). Finally, in 2010, Ricucci and Siqueira (70) concluded that overall findings are consistent with acceptable criteria to include apical periodontitis in the set of biofilm-induced diseases. In short, biofilm is a community of all different types of microorganisms that adhere to surfaces frequently embedded in a self-produced matrix of extracellular polymeric substance (EPS). This type of infection is extremely difficult to destroy with antibiotics because of the EPS, but it can be totally destroyed with NaOCl at the proper concentration and volume.
In 2006, Clegg et al. (29) demonstrated that wild biofilm grown in-vitro could be completely hydrolyzed in a concentration of 5% NaOCl. In 2011, Del Carpio-Perochena et al. (71) demonstrated a similar finding when the biofilm was grown in-vivo. Both studies agreed that chlorhexidine did not destroy the biofilm. Accordingly, the objective to obtaining biofilm destruction is to flow an adequate volume of greater than 5% NaOCl to the apical termination. This has proven difficult with various types of endodontic irrigation systems. For example, the SAF system is designed to concurrently deliver the irrigant during instrumentation. In a recent study by Paranjpe et al. (51) using the SAF system demonstrated that after instrumentation, which included a final irrigation, biofilm still remained at both the 1 and 3 mm—WL level (Figure 10.8).

Figure 10.8 After SAF instrumentation and irrigation, biofilm still remained vital and apparent on the walls of the test group.
It is necessary to further emphasize that biofilms have developed several other mechanisms against disinfecting methods; at mature stages of biofilm growth, these mechanisms are stronger. Shen et al. (72) demonstrated the importance of this factor and according to their analysis, at day 21 of growth, biofilms develop certain defense characteristics and observed differences in their architecture between young and old stages. Results c/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


