Preventive and Bleaching Materials
Chapter Objectives
On completion of this chapter, the student will be able to:
1. Describe the applications of fluoride in prevention.
2. Explain how fluoride protects teeth from caries.
3. Discuss the various methods of fluoride delivery.
4. Explain the benefit of using an antibacterial rinse in conjunction with fluoride.
5. Describe the antibacterial effects of chlorhexidine.
6. Apply topical fluoride gel or foam correctly (as permitted by state law).
7. Discuss the use of sealants for prevention of pit and fissure caries.
8. Describe the composition of sealants.
9. Recite the steps for applying sealants.
10. Apply pit and fissure sealants correctly (as permitted by state law).
11. Recite causes for tooth sensitivity.
12. List the various materials used for treating sensitive teeth.
13. Explain how desensitizing agents work.
14. Describe the uses of mouth guards.
15. List the materials for the fabrication of mouth guards.
16. Fabricate a sports mouth guard.
17. Describe the methods used to bleach teeth.
18. Discuss the similarities and differences among the materials used to bleach teeth.
20. Apply high-strength bleach on prepared teeth (as permitted by state law).
Key Terms defined within the chapter
Prevention/Preventive Aids chemicals, devices, or procedures that reduce or eliminate disease or tooth destruction in the oral cavity
Fluoride naturally occurring mineral that helps protect tooth structure from dental caries
Fluorosis enamel condition caused by consumption of excessive levels of fluoride
Demineralization action that removes mineral from the tooth, usually caused by acids
Fluorapatite tooth mineral that results when fluoride is incorporated into the tooth
Cariogenic substances or microorganisms that promote dental caries
Antibacterial Mouth Rinse liquid used to rinse the oral cavity to reduce or suppress bacteria associated with dental caries or periodontal disease
Substantivity property of a material to have a prolonged therapeutic effect after its initial use
Over-the-Counter (OTC) available in retail or drug stores without a doctor’s prescription
Sealant a protective resin that is bonded to enamel to protect pits and fissures from dental caries
Desensitizing Agent a chemical that seals open dentinal tubules in order to reduce tooth sensitivity to air, sweets, and temperature changes
Custom-Made made specifically to fit one individual
Bleaching a cosmetic process that uses chemicals to remove discolorations from teeth or to lighten them
Extrinsic Stains stains occurring on the tooth surface
Intrinsic Stains stains that are incorporated into the tooth structure, usually during the tooth’s development
Patients often ask dental assistants and hygienists about the prescription or over-the-counter (nonprescription) agents the dentist has recommended for the prevention of tooth decay and periodontal disease and for tooth whitening. In addition, the dental assistant or hygienist is often asked by the dentist to dispense, apply, or fabricate devices such as fluoride or bleaching trays and to deliver these prevention/preventive aids or cosmetic materials or devices to the patient. In many states, the dental assistant or hygienist, when properly licensed, can apply sealants prescribed by the dentist. The indications for sealants, application techniques, and troubleshooting guides are discussed. It is essential that all members of the dental team are familiar with the mechanism of action and application of these products. This chapter presents information on fluorides, antibacterial mouth rinses, sealants, desensitizing agents, mouth guards, and teeth bleaching materials. Clinical and laboratory procedures for many of these topics are included.
Fluoride
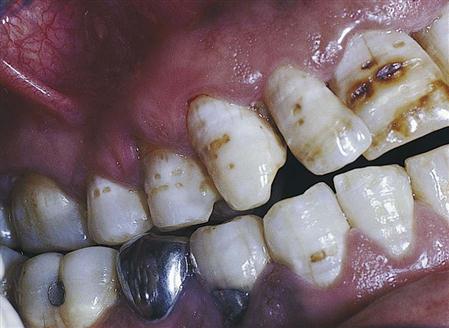
Fluoride is a naturally occurring mineral found in many forms in the modern world. It may be found in well water, in food that has absorbed fluoride from the soil, and as an additive in many dental products that we buy over the counter or that are prescribed by dentists and physicians. The accepted optimal level of fluoride in the drinking water is in the range of 0.7 to 1.2 mg/L or parts per million (ppm). Consumption of excess fluoride during formation of the teeth may lead to a condition known as fluorosis. Severe fluorosis can cause brown staining and pitting of the enamel surface (Figure 7-1) and is found where high levels (more than 2 ppm) of fluoride occur naturally in the drinking water. Mild or moderate fluorosis may create cosmetic concerns for some people by causing opaque white spots or bands on the teeth. Fluorosis is usually caused by the concentration of fluoride in the water being too high, but it may also be caused by excess fluoride toothpaste swallowed by the child or by other iatrogenic (doctor-induced) factors such as overly prescribed fluoride drops or lozenges.
Topical Versus Systemic Effects
The enamel and dentin of the tooth are composed of tiny mineral crystals (hydroxyapatite) within a protein/lipid matrix. Microscopic gaps or pores between these millions of crystals are filled with protein, lipid, and water. It is in these matrix gaps that small molecules such as lactic acid and ions such as hydrogen, calcium, and phosphate are allowed to pass. There is a constant interchange of mineral ions between the tooth surface and the saliva. Usually, the minerals entering the surface balance the minerals coming out of the tooth surface. The tooth crystals are not pure hydroxyapatite but contain carbonate, which makes them much more soluble in acid. When bacteria in the plaque on the tooth surface metabolize cooked starches or sugars, they produce acids. The acids remove more mineral (demineralization) than the amount of mineral coming into the tooth from the saliva. When these acid attacks are repeated over time, the surface becomes more porous and allows bacteria to enter the tooth. This is the start of the caries process.
For years it has been thought that fluoride incorporated into the teeth at the time of development was the main reason for the lowering of dental caries (tooth decay) rates seen in areas of water fluoridation. However, work by Featherstone and others (1990) and epidemiological studies have shown that fluoride’s greatest anti-caries benefit is gained from topical fluoride exposure after the teeth have erupted. Fluoride in the saliva surrounding the tooth is incorporated into the surface of enamel crystals during remineralization (replacing minerals lost from the tooth surface) to form a surface veneer containing fluorapatite that has much lower solubility than the original tooth mineral. The pH (a measure of acidity) at which tooth mineral dissolves is 5.5 (7.0 is neutral pH—neither acid nor base). However, when the mineral is converted to fluorapatite, the pH at which it dissolves is lowered to 4.5 (a lower number indicates that it is more acidic; e.g., stomach acid has a pH of less than 1.0). Therefore fluoride makes it more difficult for the acids produced by cariogenic (decay-causing) bacteria in plaque to demineralize tooth structure and cause dental caries. There is evidence that fluoride from drinking water, toothpastes, mouth rinses, and some foods remains in the saliva for several hours and has a prolonged topical effect.
Protection Against Erosion
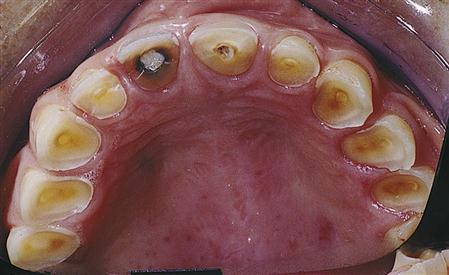
Highly acidic foods and beverages such as citrus fruits, sodas, and wine can contribute to loss of tooth mineral that is called erosion. Erosion differs from caries in that bacteria are not involved and most of the mineral loss is at the surface. It is important to maintain a well-balanced diet to minimize excess acidic foods. Some medical conditions also cause erosion of the teeth by causing stomach acid to enter the mouth. Examples are acid reflux (burping up stomach acid), anorexia (body wasting from extreme dieting and forced vomiting to purge food and keep from gaining weight) and bulimia (chronic forced vomiting to control weight gain after binge eating) (Figure 7-2). By making the tooth structure less soluble in acids, fluoride provides some degree of protection against erosion, but repeated acid attacks will overcome the beneficial effects of fluoride.
Bacteria Inhibition
Fluoride interferes with the essential enzyme activity of the bacteria. Although the fluoride ion has been shown not to cross the bacterial cell wall, it can travel through it in the form of hydrofluoric acid (HF). As the decay-causing bacteria produce acids during the metabolism of sugars and cooked starch, some of the fluoride present in the plaque fluid combines with the hydrogen ion of the acid to become HF and rapidly diffuses into the cell. Once in the alkaline cytoplasm of the cell, the HF again separates into the fluoride ion and the hydrogen ion. These ions disrupt the enzyme activities essential to the functioning of the bacteria and cause their death.
Fluoride and Antibacterial Rinses for Control of Dental Caries
Studies have shown that fluoride alone is not as effective in managing dental caries as when it is used in conjunction with an antibacterial mouth rinse. Therapeutic mouth rinses help suppress bacteria associated with dental caries but are not meant to be substitutes for daily mechanical plaque removal.
Chlorhexidine gluconate is a bis-bisguanide that is effective against a broad spectrum of microorganisms. In several European countries, it is used at a concentration of 0.2%, but in the United States, the maximum concentration allowed by the U.S. Food and Drug Administration (FDA) is 0.12%. It is a prescription mouth rinse that is available commercially through several companies. The most common trade names are Peridex, Periogard, and Oris CHX. It is one of the most effective agents for reduction of plaque (55%) and gingivitis (45%). Chlorhexidine kills bacteria by binding strongly to the bacterial cell membrane, causing it to leak and lose its intracellular components. It binds very strongly on many sites in the oral cavity, including the mucous membranes and plaque, and is released slowly, giving it a prolonged effect (called substantivity). The antibacterial effect from a single dose is greatest for several hours after use, but it may last for a few days. It is used in the management of many bacteria associated with periodontal disease and also is effective in suppressing mutans-type Streptococci associated with dental caries. The currently recommended rinsing regimen to control dental caries as suggested by an organization of western U.S. dental schools is as follows: Rinse nightly for 1 minute with approximately 10 ml of 0.12% chlorhexidine for 1 week each month. Repeat the cycle monthly until it has been determined by the dentist who is using bacterial cultures of mutans-type streptococci and lactobacilli that further rinsing is not needed.
The side effects associated with this product are the formation of a brown stain on the teeth and tongue; on glass ionomer, compomer, and composite restorations; and on artificial teeth. It has a bitter taste and may affect the taste of some foods. Staining seems to be more rapid in some individuals. Diet and brushing habits are thought to play an important role in how rapidly staining occurs. Some flavoring agents have been introduced to offset the bitter taste. The solution contains alcohol that might be harsh for individuals with sensitive mucous membranes. A nonalcohol version is now available. More frequent professional teeth cleaning and polishing is usually necessary for patients who use these compounds routinely.
The longest-used antibacterial mouth rinse agents are the phenolic compounds also called essential oils. The best-known product is Listerine, which has received the American Dental Association (ADA) Seal of Acceptance. It is a combination of phenol-related essential oils (thymol, eucalyptol, and menthol) mixed in methylsalicylate in a 26.9% hydroalcoholic vehicle. The antibacterial action of these compounds is a result of their alteration of the bacterial cell wall. It has not been shown to be an effective anti-caries rinse, but clinical studies have shown reduction of plaque scores by about 25% and gingivitis by 30% with use of these compounds. In some patients, these compounds cause a burning sensation in the tissues and a bad taste. Flavoring agents have been added to try to overcome the taste problem.
Methods of Delivery
Dietary Fluoride Supplements
Fluoride may be obtained through the drinking water, either naturally occurring or in fluoridated water supplies. In nonfluoridated communities, dentists and physicians may prescribe fluoride supplements for children in the form of tablets, drops, or lozenges. Consideration should be given to the total fluoride exposure the child receives from other sources, such as school rinse programs, toothpaste, or prepared foods with fluoride. There is a schedule that recommends daily doses of fluoride supplements based on the child’s age and the fluoride content of the community water supply. Supplements are not desired when the community water supply has a fluoride content that is higher than 0.6 ppm. Tablets and lozenges should be sucked to gain a topical effect. A portion of systemically ingested fluoride, including that in drinking water, is returned to the oral cavity by way of the saliva, thereby contributing to a topical effect.
In-Office Fluoride Applications (Topical)
Children with newly erupted permanent teeth and children and adults at high risk for caries are good candidates for professionally applied fluorides. The dental hygienist is most often the professional applying the fluoride in conjunction with a dental prophylaxis. In some states properly trained dental assistants can also play this important role. The most commonly used fluorides come in the form of topical gels or foams that are applied for 4 minutes in disposable trays. Some manufacturers market topical fluorides that they suggest need be applied for only 1 minute. A 1-minute application is not recommended by the ADA. The 1-minute application delivers approximately 85% of the fluoride that a 4-minute application delivers. However, a 1-minute application has an appeal to clinicians who have to manage small children who have active tongues and profuse salivary flow, and patients who tend to gag easily. When used one to two times a year, topical fluoride treatments have been shown to produce 20% to 26% caries reduction. Acidulated phosphate fluoride (APF) is most often used with children, because it contains 12,300 ppm fluoride and has good uptake in the enamel. Two percent neutral sodium fluoride (NaF) contains 9000 ppm fluoride and is used more often with adults, because the acid in APF tends to etch the surface of restorations made of porcelain, composite resin, glass ionomer, or compomer. Fluoride varnishes are available as 5.0% sodium fluoride and are applied directly to the surfaces of the teeth. They remain on the teeth for 1 to 3 days if the patient brushes gently. They are particularly useful for direct application to early dental caries that can remineralize (Figure 7-3). Fluoride varnishes are quickly replacing the use of foams and gels in the dental office, because they can be applied rapidly and do not have the unpleasant side effects of nausea, vomiting, and gagging often seen with tray application of foam or gel.
Self-Applied Topical Gels
Self-applied fluoride gels are recommended for individuals who are at moderate to high risk for dental caries. They are also used for orthodontic patients to prevent caries and decalcification around brackets and bands that causes permanent white spots and lines on the enamel. These white spots are unsightly and represent the early stages of the caries process. Elderly patients who take medications that dry up their salivary flow are at high risk for root caries and can receive benefit from gels used at home. Self-applied gels are available by prescription as 1.1% neutral sodium fluoride (5000 ppm fluoride) or 0.4% stannous fluoride (900 ppm fluoride). Stannous fluoride may cause some staining of the surfaces of the teeth, and it delivers less fluoride ion to the teeth. These gels can be brushed on the teeth or applied in custom fluoride trays. Four minutes of use in a custom tray is much more effective than 1 minute of brushing with the gel, because the saliva quickly dilutes the gel and removes it from contact with the teeth. The custom trays, however, involve some additional expense for the time required to make impressions, pour casts, and construct the trays. Children younger than 6 years of age should not use these gels, because they tend to swallow too much of the gel. In place of a brush-on gel, some manufacturers have made a prescription toothpaste containing 1.1% neutral sodium fluoride. The idea is that it will aid compliance, because the patient will not have to brush the teeth first and then brush again with a gel, but can achieve both at the same time.
Over-the-Counter Fluoride Rinses
Over-the-counter (OTC) fluoride rinses have been demonstrated to provide 28% caries reduction when used in a daily rinse program. Rinses are available as 0.05% sodium fluoride (225 ppm fluoride) (Figure 7-4). Patients typically are instructed to rinse for 30 to 60 seconds, spit out the excess, and not eat or drink anything for at least 30 minutes. It is often used just before bedtime so that a residue of fluoride can remain in the saliva during sleep. Prescription rinses contain 0.2% sodium fluoride or 0.63% stannous fluoride.
Fluoride-Containing Toothpaste
Studies with Crest toothpaste conducted in the 1950s first established the caries-preventive capability of fluoride in toothpaste. Many studies conducted since then have shown sodium monofluorophosphate (MFP) and sodium fluoride to be more effective and chemically stable than stannous fluoride. The fluoride content of most toothpaste is about 1000 ppm. Children younger than 6 years of age should be supervised when brushing and should be given only a pea-sized amount of toothpaste once a day. Toothpaste for children is available with a much lower fluoride content. They tend to swallow the paste and run the risk of mild fluorosis of the permanent teeth if they consume too much.
Fluoride-Containing Prophylaxis Pastes
Prophylaxis pastes contain pumice as an abrasive to remove surface stains and plaque from the teeth. In the process, they remove a small amount of the fluoride-rich enamel surface. It is thought that some of the lost fluoride can be regained by incorporating fluoride in the paste. The most common fluoride additive is 1.23% APF. These pastes have not received the ADA Seal as effective for caries prevention, because studies have not shown them to be effective for this purpose. In some dental offices, polishing the teeth after prophylaxis is not routinely done to avoid removing that fluoride-rich surface layer.
Safety
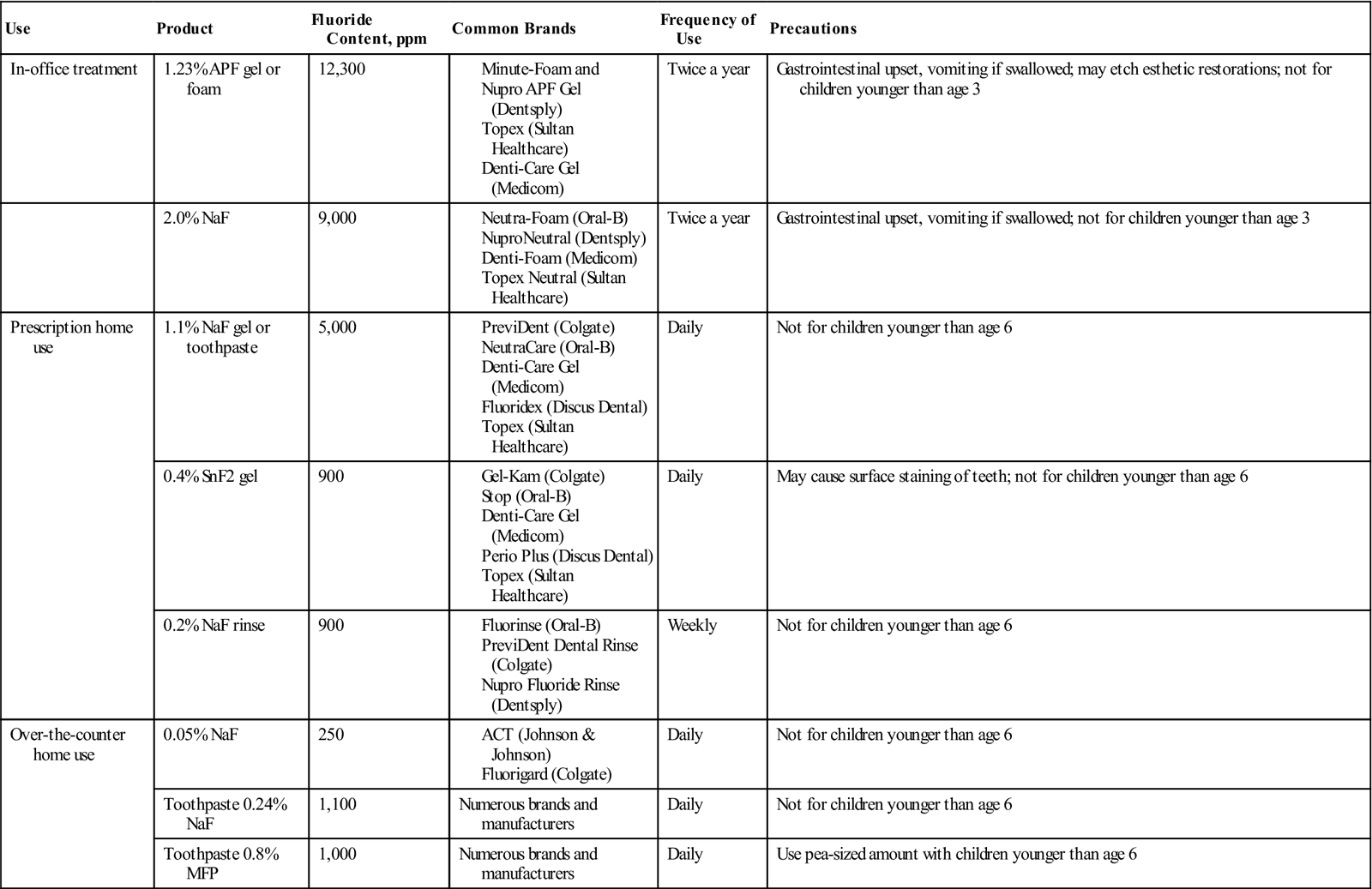
All fluorides should be used as directed and kept well away from small children for safety reasons. The lethal dose for a child weighing 20 pounds is approximately 700 to 1500 mg of sodium fluoride. Therefore, it is recommended that prescriptions for dietary supplements of fluoride contain no more than 120 mg to avoid the risk of a fatal overdose. Dental assistants and dental hygienists should realize that overdoses causing acute illness can occur from topical applications of fluoride as well. If it is determined that a child has consumed an excessive amount of fluoride, vomiting should be induced and milk of magnesia should be given to tie up the fluoride. Cow’s milk could be given to slow absorption from the stomach. The child should be taken to the emergency room of the nearest hospital. The most common reaction seen in the dental office or shortly after leaving the office when a child has swallowed fluoride gel is nausea and vomiting. The fluoride, particularly with the acidulated gel, irritates the stomach. (See Procedure 7-1 at the end of this chapter for the clinical technique for in-office topical fluoride application.) Table 7-1 lists common in-office and home-use fluoride products.
TABLE 7-1
Common In-Office and Home-Use Fluoride Products
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Pit and Fissure Sealants
Purpose
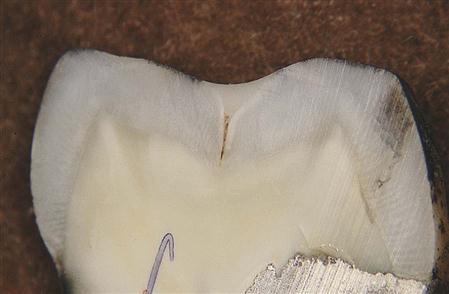
Sealants are unfilled or lightly filled resins that are used to seal the noncarious pits and fissures of deciduous and permanent teeth (see Chapter 6). The purpose of a sealant is to prevent dental caries in the pits and fissures. The widespread use of fluoride has caused a significant reduction in dental caries in children who receive regular dental care, but not necessarily in low-income children. Although the overall caries rate has dropped, the greatest benefit from fluorides has been seen on smooth enamel surfaces. Most caries (about 88%) in children is found in pits and fissures. The nature of the shape of the pits and fissures makes them vulnerable to dental caries. Pits and fissures are often deep, narrow channels in the enamel surface that can extend close to the dentinoenamel junction (Figure 7-5). They collect bacteria and food debris that cannot be removed by toothbrushing, so dental caries can occur readily in these locations. Sealants are not as widely used as they should be. Among low-income children, utilization of sealants is only about 30%, and this is the high caries risk group that could benefit most from sealants.
Sealants have been shown in numerous studies to be an effective and conservative means of preventing caries in pits and fissures by preventing bacteria and food products from entering them. Caries is often difficult to detect in its early stages in pits and fissures. For this reason, some dentists are reluctant to use sealants for fear of sealing undetected caries. There is ample evidence in the dental literature that if caries is inadvertently sealed in the pits and fissures, the caries process stops, because bacteria are cut off from their nutrients. Going and his coinvestigators found mostly negative bacterial cultures in teeth with carious dentin that has been sealed with Nuva-Seal for 5 years. Treatment of carious teeth with sealants resulted in an 89% reversal from a caries-active to a caries-inactive state. Those sites that remained carious had significantly fewer viable bacteria than unsealed carious control sites. Handelman and his coinvestigators demonstrated a decrease of 2000-fold in the bacteria that could be recovered from carious dentin in pits and fissures that were sealed for 2 years compared with unsealed control teeth. They demonstrated that small caries inadvertently sealed in the tooth and followed on radiographs for 2 years not only did not progress, but in some cases repaired partially. However, only incipient enamel caries should be considered for the sealant procedure. If a sealant leaks because it is not properly placed, caries can occur beneath it. More advanced caries should be treated with conservative restorative procedures rather than with sealants.
Indications
The lack of an accurate means of predicting where caries will occur has complicated the process of selecting which teeth should be sealed and which should not. Because some individuals will remain caries-free throughout their lifetime, it is not indicated to seal all posterior teeth. The dentist should use his/her clinical judgment based on specific criteria to determine which teeth should be sealed. Consideration should be given to the age, oral hygiene, caries risk, diet, fluoride history, and tooth type and morphology.
Although the main thrust of sealant therapy is aimed at permanent teeth, primary molars may also be sealed to reduce the caries rate and prevent premature tooth loss. Approximately 44% of caries in primary teeth occurs in the pits and fissures of the molars. Although the occlusal morphology of primary molars is flatter and less fissured than that of permanent molars, sealants are still indicated if deep or stained fissures are found.
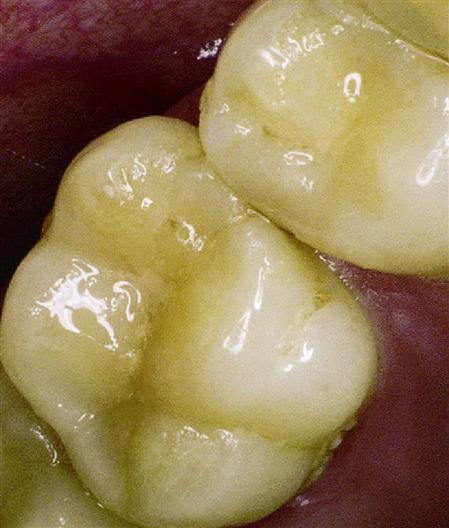
Permanent teeth should be sealed if there is/was evidence of caries susceptibility in the primary dentition. Teeth with steep cuspal inclines and deep, sticky fissures are more likely candidates for sealants than teeth with shallow cusps and highly coalesced (fused together) pits and fissures (Figure 7-6). Molars decay three to four times more frequently than premolars, undoubtedly as a result of the more complex occlusal morphology. Premolars are not generally high-risk teeth, and sealants should be applied selectively when specific indications are present. Occasionally, maxillary central and lateral incisors have deep lingual pits that require sealing. However, emphasis is placed on sealing first and second molars as a priority.
Susceptibility of Teeth to Fissure Caries
Teeth most susceptible to pit and fissure caries are listed in the order of their risk for decay:
• Lower molars—about 50% of the caries occurs in these teeth
• Upper molars—about 35% to 40%
• Upper and lower second premolars
Taken as a group, caries occurs most often in upper and lower molars, accounting for 85% to 90% of the pit and fissure caries.
Composition
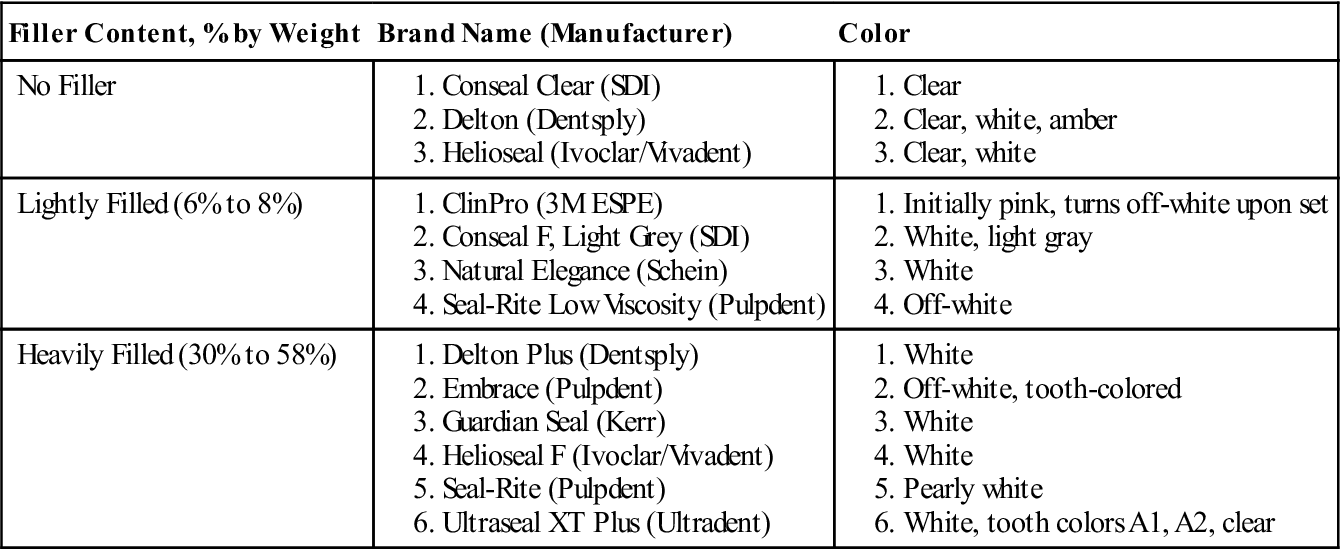
Sealants are chemically similar to composite resins. Their resin component is based on a dimethacrylate monomer that is either bisphenol A-glycidyl methacrylate (bis-GMA) or urethane dimethacrylate (UDMA). Polymerization of the resin occurs either solely by chemical reaction (self-cure) or by light activation (light-cure) (see Chapter 6). The self-cure is by the conventional peroxide-amine system, which requires the mixing of two components. The light-cured sealants are one-component systems that use blue light to polymerize them. A vast majority of sealants in use today are light-cured. Many manufacturers add very small filler particles to the sealants to make them more wear resistant. Sealants are not as heavily filled (see Table 7-2 for sealant filler content) as most composites, because they would be too viscous to flow into the narrow fissures. Some of the filler particles used in sealants may be radiopaque and may allow the sealants to be seen on x-rays. Many sealants, however, are radiolucent (see Chapter 6).
TABLE 7-2
Filler Content and Color of Commercial Sealants
< ?comst?>
| Filler Content, % by Weight | Brand Name (Manufacturer) | Color |
| No Filler | ||
| Lightly Filled (6% to 8%) | ||
| Heavily Filled (30% to 58%) |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Working Time
The self-cured sealant polymerizes to final set within approximately 2 minutes from the start of mixing of the two components, one with the initiator and the other with the accelerator. An experienced operator can apply the material to one or two quadrants of posterior teeth with one mix of material, so it has the advantage of being applied faster than the light-cured material on a comparable number of teeth. The light-cured material requires a 20-second application of light on each tooth to polymerize the sealant if a standard halogen light is used. LED and laser curing lights can be much more intense and require less curing time. Use the manufacturer’s recommendations for curing times. The light-cured material has the advantages of not requiring mixing, which often incorporates bubbles into the material, and allowing the operator to place and cure the material when the operator is ready.
Color and Wear
Manufacturers provide sealants in a variety of colors. Sealants may be clear, amber, tooth colored, or opaque white. Patients usually prefer the clear or tooth-colored sealants, but it is easier for the dental team to identify the presence of the sealants at the time of placement and at subsequent examination visits if they contrast with the tooth color. Sealants are subject to wear from the occlusion. Sealants that contain no organic filler particles will wear faster than those that have filler particles added. Some clinicians use flowable composites as sealants, because they are more heavily filled and therefore are more resistant to wear while at the same time having adequate flow to enter the fissures. Sealants seldom flow to the bottom of long, narrow fissures because of the presence of debris (see Figure 7-5) and trapped air. Some clinicians prefer to open the fissures with a small-diameter bur or diamond to look for decay, remove debris, and allow better penetration of the sealant. Wear does not create much of a problem as long as the fissure remains sealed. If part of the fissure is uncovered, repair is recommended.
Placement
The ADA Council on Scientific Affairs does not recommend routine opening of fissures with cutting instruments prior to sealant placement. The technique of placement of sealants requires attention to detail (see Procedure 7-2 at the end of this chapter). This technique has many steps in common with the placement of other bonded restorations (see Chapters 5 and 6). The surface must first be cleaned with pumice to remove any surface debris that would interfere with acid etching or bonding. Retention of the sealant is obtained by etching the enamel with 37% phosphoric acid to roughen it and to open pores in the enamel for penetration of the resin sealant. After etching, rinsing, and drying of the enamel, isolation of the field is very important. Etching enlarges the size and volume of pores in the enamel so that the sealant can penetrate and mechanically lock into these spaces. Some clinicians like to use a drying agent after etching and rinsing to remove any remaining water in the fissures held there by capillary action. Drying agents usually consist of alcohol. They will mix with the water, and when they evaporate, they will carry off the excess water with them. Studies have shown that application of an enamel bonding resin prior to placement of the sealant enhances the retention and seal. Bonding resins are low-viscosity resins that can flow readily into the fissures and into the microscopic porosities created by acid etching. The resin-containing sealant will then bond to the bonding resin by a chemical resin-to-resin bond. However, many clinicians have not yet started using bonding agents with sealants, because this is a relatively new finding. The sealant is applied to the pits and fissures and surrounding enamel and is cured.
Oxygen-Inhibited Layer
The cured sealant will have a very thin film of uncured resin on its surface. The surface will appear shiny and will be wet to the touch, because the set of the resin at its surface is inhibited by contact with oxygen in the air. This film is called the oxygen- or air-inhibited layer. It causes no harm but should be wiped off with gauze or a cotton roll, because it might have an unpleasant taste to the patient.
Remineralization of Etched, Unsealed Enamel
One concern that was raised about etching enamel surfaces for placement of sealants is that if the sealant comes off, the exposed surface is more caries susceptible. Studies have shown that the etched enamel begins remineralization after a 24-hour exposure to saliva by deposition of calcium phosphate salts. In areas where sealants wear away, resins tags remaining in the enamel provide some caries protection.
Etching Precautions
Care should be taken in placement of the acid etchant so that adjacent teeth are not etched and the soft tissues are not exposed to the acid. Mylar matrix strips or metal matrix bands can be placed in the interproximal spaces to prevent etching of adjacent teeth, but careful application of etchant will prevent this from occurring. Care should also be taken to avoid contact of the acid with the eyes and skin of the patient and operator. Both should wear protective eyewear.
Bite Interference by Sealant
If a sealant is too high, it might cause interference with the bite of the patient. Unfilled sealants that are too high will wear down in a few days or weeks. Sealants with filler particles are much more wear resistant. Ideally, all high sealants should be adjusted to be compatible with the patient’s bite. Otherwise, sore teeth or jaws may result. Articulating paper should be used to identify the high spots, and an appropriate bur or diamond can be used to adjust them.
Patient Record Entries
The sealant procedure should be carefully documented in the patient’s chart. Chart entries should include the following:
Studies have shown that retention rates are better when four-handed techniques are used for the placement of sealants. Having an extra pair of hands to help maintain isolation and place or cure the sealants is a definite bonus. In many states, dental hygienists and dental assistants can be licensed to place sealants. State practice act guidelines must be followed as to the oral health care providers permitted to place sealants and adjust the occlusion on a high sealant. The dental hygienist can play an important role in the maintenance of sealants by carefully checking them at hygiene visits.
Effectiveness
Carefully placed sealants are very effective at preventing decay in the pits and fissures. Simonsen (1991) followed sealants for 15 years after placement and found them to be highly effective. In that study, sealants were placed on permanent posterior teeth; this procedure was followed by periodic examinations. If sealants were completely or partially lost, they were not replaced or repaired. (In dental practice, lost sealants would be replaced.) At the end of 15 years, more than 68% of teeth were caries free compared with 17% in a control group with no sealants. A much greater reduction in caries could have been obtained by replacement of lost sealants.
Troubleshooting Problems With Sealants
Most failures occur within the first 3 to 6 months, and all or part of the sealant comes off. The worst failure is a sealant that leaks but remains in place. The leak can go undetected and can decay significantly underneath the sealant before it is detected. Placing too much sealant can result in excess mater/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses