Principles of Bonding
Chapter Objectives
On completion of this chapter, the student will be able to:
1. Discuss the effects of acid etching on enamel and dentin.
2. Describe the basic steps of bonding.
3. Explain the differences between bonding to enamel and bonding to dentin.
4. Describe the agents used for bonding.
5. Explain the differences between total-etch and self-etch bonding techniques.
6. Explain how the hybrid layer is formed.
7. Discuss the factors that interfere with good bonding.
8. Describe the amalgam bonding technique.
9. Describe the bonding of orthodontic brackets.
10. Describe the bonding of endodontic posts.
11. Explain the differences in bonding to enamel, dentin, metal, and porcelain.
12. List the factors that contribute to tooth sensitivity after bonding.
KEY TERMS defined within the chapter
Etching or Conditioning terms used interchangeably to describe the process of preparing the surface of a tooth or restoration for bonding. The most common etching material (etchant) is phosphoric acid
Wet Dentin Bonding bonding to dentin that is kept moist after acid etching to facilitate penetration of bonding resins into etched dentin
Hydrophilic an attribute that allows a material to tolerate the presence of moisture
Hydrophobic an attribute that does not allow a material to tolerate or perform well in the presence of moisture
Total-Etch System a bonding system that includes etching of both enamel and dentin as a separate step from the application of bonding agents
Self-Etch System a bonding system that does not use a separate etching procedure with phosphoric acid. The acid is contained in the resin primer and no rinsing is needed
Hydrodynamic Theory of Tooth Sensitivity pain caused by movement of pulpal fluid in open (unsealed) dentinal tubules. Actions that cause a change in the pressure on the fluid within the dentinal tubules stimulate a pain response from nerve fibers in the odontoblastic processes that extend into the dentinal tubules from the pulp
The modern dental practice uses bonding for a wide variety of dental procedures. The dental assistant and the dental hygienist must be familiar with the terms and processes used in bonding of various restorative and preventive materials, to be knowledgeable, effective members of the dental team. The dental assistant will be involved in helping the dentist perform bonding procedures many times each day, and in some states may perform the etching of tooth structure and the application of bonding agents and placement of sealants and composite resins. The dental hygienist may place sealants and perform prophylaxis procedures that might affect bonded restorations. Therefore, it is important that the allied oral health practitioner understand the properties and handling characteristics of the bonding materials and the processes involved in their use.
Basic Principles of Bonding
In dentistry, the term bond, or bonding, is used to describe the process of attaching restorative materials, such as a bonded amalgam or a bonded composite resin, to the tooth by adhesion (attraction of atoms or molecules of two different contacting surfaces). When describing cosmetic restorations such as porcelain or composite veneers, patients often use the term bonding, for example, “The dentist is bonding my front teeth.” Bonding also is the basis for several other dental procedures, such as the placement of resin-bonded bridges and orthodontic brackets and fixed retainers. It is used to describe some of the materials used in the process of placing restorations. For example, bonding resin is placed on the etched tooth surface before light-curing. Manufacturers often use the word “bond” in the trade names of their bonding resins, such as Prime & Bond NT (Dentsply International, York, PA).
Preparation for Bonding
The first step in the bonding process involves preparation of the surface of the tooth or the restoration (or both) to receive the material that will be bonded to it. Preparing the tooth surface usually includes removing plaque and debris, then etching or conditioning the enamel or dentin (or both) with an acid. The most commonly used acid is phosphoric acid in concentrations ranging from 10% to 38%. Acid removes mineral from the surface to create roughness or microscopic porosity.
Bonding to the Etched Surface
When a resin bonding agent or primer is flowed over the etched surface, it penetrates into the microscopic pores. When it hardens (cures or polymerizes), it creates projections called resin tags that lock into the tooth, creating a mechanical bond called micromechanical retention. The resin bonding agent will then chemically bond to other resins placed over it, such as composite resin. The chemical bond, called a primary bond, is a true adhesion between atoms or molecules of the composite resin and the bonding resin. The chemical bond is stronger than a physical bond, called a secondary bond, which is a weak physical attraction between two surfaces such as the adhesion of paint to a metal surface. Roughening the metal surface by sandblasting increases the adhesion of the paint by mechanical retention, much in the way that acid etching roughens the surface of the enamel.
Surface Wetting
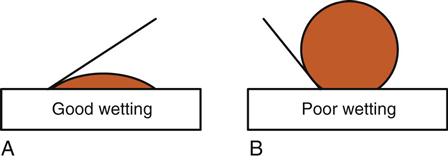
Acid etching also increases the ability of liquids to wet the surface of the tooth by creating a high surface energy. High surface energy can attract contaminants (such as saliva), so good isolation is important. If saliva contamination occurs, drying the surface will leave residues that will interfere with the bond. The surface must be re-etched. Good wetting increases the intimate contact of the bonding resin with the etched tooth structure, improving the penetration of resin to form tags and thereby improving the bond. High surface energy helps to attract the resin to the etched surface. Surfaces that are poorly wet will cause beading of the liquid, similar to water on a newly waxed car. The bead of water stands up on the surface of the car with a high angle of contact. On an unwaxed car, the water easily spreads out and has a low angle of contact (Figure 5-1). Bonding agents are usually not very viscous (thick), so they will flow readily and wet the etched surface.
Bond Strength
The strength of the bond obtained is usually measured by determining the force needed to separate the two joined materials. The force needed to break the bond is divided by the cross-sectional area of the bonded surfaces to arrive at the value for the bond strength. Most bond tests pull the bonded materials apart (tensile bond strength) or apply forces at approximately 90 degrees to the bonded interface of the materials until the bond fails (shear bond strength). The value for the bond strength is reported as MPa (MegaPascals). One MPa equals 150 psi (pounds per square inch). Choosing materials with good bond strengths to tooth structure can enhance the longevity of the restoration and potentially allow for more conservative preparations, because cutting into healthy tooth structure to create mechanical locks can be minimized.
Bonding to enamel usually achieves consistently high bond strengths of around 30 MPa (4500 psi). The bond strength to dentin is usually less than to enamel and varies according to how deep into the dentin the cavity preparation extends. The dentin near the dentinoenamel junction (DEJ) has fewer dentinal tubules (about 15,000 to 20,000/mm2), occupying 14% of the dentin surface, and they are smaller in diameter than in the dentin closer to the pulp. Deeper dentin contains more tubules (about 45,000 tubules/mm2) and they are larger in diameter, occupying 20% to 30% of the dentin surface. Fluid flows from the pulp into the tubules on a constant basis. Therefore, deeper dentin will be wetter dentin from the flow of pulpal fluid through the tubules. Wetter dentin with more holes (tubules) is more difficult to bond to consistently than is shallower dentin. Over time, exposure of the bonding agents to moisture may cause them to degrade (hydrolyze). In addition, repeated stresses on the bond caused by chewing pressures and temperature changes that cause different expansion and contraction amounts between the restoration and the tooth structure (measured by the coefficient of thermal expansion) will gradually cause fatigue failure of the bond. (Fatigue failure is similar to taking a piece of metal and repeatedly bending it back and forth until it breaks.) When composite resin is placed and polymerized, it shrinks and can put stress (as much as 20 MPa or 3000 psi) on the bond of the resins to the tooth. In addition, hot and cold foods or beverages can cause composite resin to expand and contract much greater than the tooth (about four times greater). If the bond fails, the restoration could leak, causing sensitivity in the tooth or leading to recurrent caries.
Enamel Etching
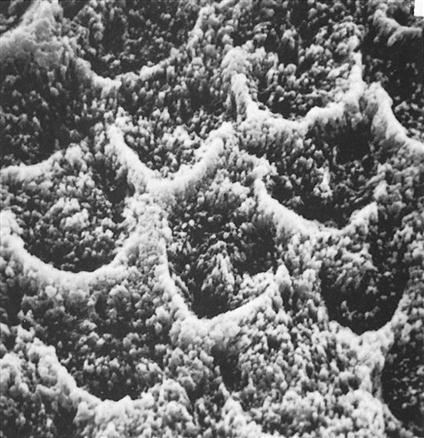
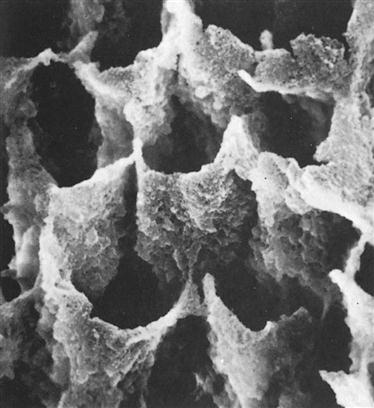
Dr. Michael Buonocore introduced etching of enamel into dentistry in the 1950s. Enamel is composed of thousands of rods (prisms) that extend from the dentin to the tooth surface in a radial fashion. Each rod has many millions of crystals composed of hydroxyapatite that has about 20% carbonate inclusions. These carbonate imperfections add to the solubility of the crystals in acid. Proteins, lipids, and water in small quantities are found in microscopic spaces between the crystals. Etching of enamel removes a small portion of the surface, reduces the ends of the enamel rods, and opens porosities between adjacent rods (Figure 5-2).
Etching Times
The enamel of permanent teeth is usually etched for 20 to 30 seconds with 37% phosphoric acid. Although etching times as short as 10 seconds appear to give good clinical results in some teeth, some research results suggest that 20 to 30 seconds is optimal. Some highly mineralized teeth may be more resistant to etching and may require up to 60 seconds of etching. The etched surface should have a frosty appearance when dried (Figure 5-3). However, when a cavity preparation involves the etching of both enamel and dentin, and the preparation is left slightly moist for wet dentin bonding, it cannot be determined whether the enamel has a frosty appearance. Primary teeth should be etched for longer periods (60 seconds or more) because the surface of the enamel has a prism pattern that is not as well structured, is considered aprismatic (without a regular prism pattern), and is more resistant to deep resin tag formation.
Etchant Liquid or Gel
The acid etchant comes in a liquid and a gel. Often coloring agents are added so the practitioner can see where the etchant is on the tooth. Liquid etchants are usually applied with a brush, a small cotton pellet, or a small sponge. Gels are more popular because they stay in place, whereas liquids tend to run without control. Gels contain silica as a thickener. They are usually applied by brush or dispensed from a syringe through a fine needle or brush tip. The recommended rinsing time for acid gels is approximately 10 seconds or longer. Rinsing times shorter than 5 seconds may not remove residual silica. Rinsing times for liquid etchants can be shorter—5 to 10 seconds.
Dentin Etching
Smear Layer
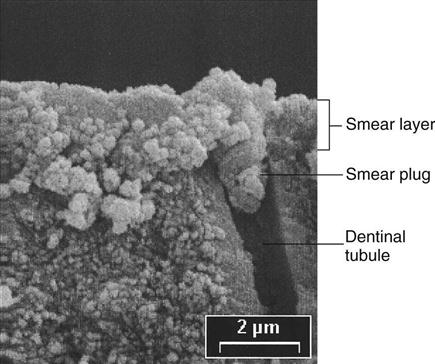
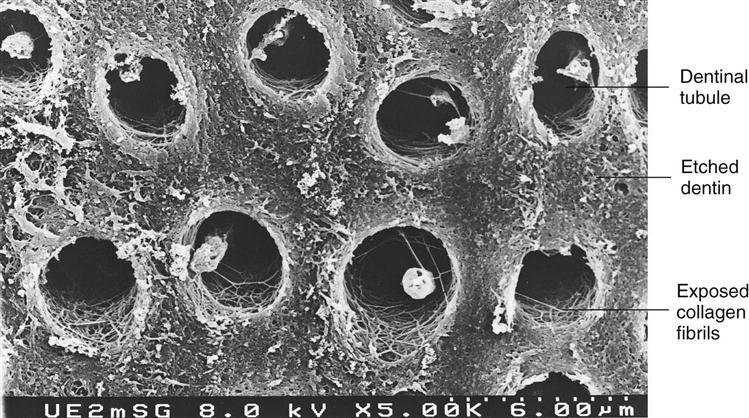
The dentin has a higher water and organic content (about 50% by volume) than does enamel (only about 13% by volume). It contains a collagen matrix woven throughout the mineral component (hydroxyapatite) and a system of dentinal tubules through which fluids from the pulp flow. When a cavity preparation is cut with rotary or hand instruments, a layer of cutting debris forms on the surface of the cut dentin and enamel. This layer, called the smear layer, is composed mostly of cut tooth structure and may also contain plaque, bacteria, pellicle, saliva, and even blood (Figure 5-4). The smear layer sticks tenaciously to the surface, plugs the openings of dentinal tubules, and cannot be washed off with use of an air-water spray. The smear layer is about 2 µm thick, interferes with formation of a bond to dentin, and needs to be removed.
Acid Etching
Etching dentin with phosphoric acid dissolves the smear layer first, then portions of the hydroxyapatite crystals from the surface of the dentin, creating a porous surface and exposing collagen fibrils that are part of the dentin matrix (Figure 5-5). Mineral is removed up to 5 µm in depth from the area between the tubules (intertubular dentin) and from around the periphery of the tubules (peritubular dentin) as well as in the opening of the tubules. Acid that goes into the tubules is neutralized by the fluids that flow from the pulp. When mineral is removed, it leaves a roughened, porous surface (but not the same as with enamel, because there are no rods or prisms). Because dentin is not as highly mineralized as enamel, it should be etched for shorter periods, typically for 10 seconds. When one is etching both enamel and dentin as in a coronal cavity preparation, it is best to apply the acid to the enamel first for 10 seconds, then to the dentin for 10 seconds. That way enamel will be etched for a total of 20 seconds and dentin only 10 seconds. Etching dentin for 20 seconds or longer opens the tubules too wide and removes hydroxyapatite mineral to too great a depth. Over-etching will expose too much collagen matrix, causing it to act as a thick barrier and making it more difficult to coat the dentin and seal the tubules with the resin bonding agents. Over-etching dentin can result in a weaker bond and in posttreatment sensitivity. The acid is removed by rinsing for at least 10 seconds. The excess water is removed by a gentle stream of air. However, the dentin is left slightly moist so that it glistens but without any puddles of water. It is critical at this stage not to over-dry the dentin. The dentin surface must be moist to keep the collagen fibrils fluffed up. If the dentin is dried too much, the collagen fibrils collapse and form a dense surface that occludes the tubules and blocks adequate penetration by the dentin bonding resins. Incomplete sealing of the dentinal tubules occurs and a much weaker bond results because the dentin/resin interface will fracture more easily. A good dentinal seal helps eliminate bacterial leakage and postoperative sensitivity.
Bonding Agents
Enamel Bonding Resins
Bonding agents are low-viscosity resins that flow well into the microscopic porosities and irregularities of the etched surfaces. When bonding to enamel alone, the process is much simpler than bonding to dentin. Etching of enamel creates a high-energy, low-tension surface that makes the surface easier to wet. Bonding to enamel alone requires only a low-viscosity liquid resin monomer that will penetrate into the spaces on and between enamel rods created by acid etching. The liquid may be simply an unfilled resin or may include small amounts of very fine filler particles to enhance the strength of the resin. A high-energy surface attracts the atoms in the resin bonding agent to improve penetration into the porous, etched enamel. When the resin is cured by a chemical process or by light activation, it locks into the microscopic spaces and irregularities, producing resin tags that can be 10 to 50 µm long (Figure 5-6). The resin tags secure the resin to the enamel and create a very strong bond (more than 20 MPa shear bond strength). The length of the resin tags in part is determined by the orientation of the etched enamel rods. If the enamel on the surface of the tooth is etched (as with bonding orthodontic brackets), the rods are etched on their ends and the resin penetration is deep. In a Class I cavity preparation, the sides of the enamel rods on the walls of the preparation have been exposed. When the sides of the rods are etched, the penetration of the resin is much shallower (about 5 to 10 µm long). Even though there is a difference in the length of the resin tags between end-etched rods and side-etched rods, the bond strengths are not significantly different. Contaminants on the surface, such as saliva or blood, can dramatically lower the strength of the bond to the enamel. This is one major reason why good isolation is so important. If enamel becomes contaminated after etching, it must be re-etched for 10 to 15 seconds before the bonding process is continued. Because most restorative procedures involve etching both enamel and dentin, a single bonding resin that can be used on both the etched enamel and dentin is preferred.
Dentin Bonding Resins
Bonding resins can be viewed as two components. The first is a resin primer that penetrates etched dentin and enamel and lays down a resin layer. The primer is composed of monomers and molecules that allow it to penetrate water (hydrophilic properties). Second, an adhesive resin is applied over the primer and the two resins chemically bond to each other, that is, the initial resin bonding material prepares (or primes) the tooth surface, much in the way that a primer is applied to wood before painting so the paint will adhere better. The second resin then chemically bonds to the primer. In an effort to simplify the bonding technique, many manufacturers have combined the primer and bonding resin into one bottle to eliminate one step in the process.
Currently, bonding to moist dentin is the accepted technique (called wet dentin bonding). The dentin is kept moist to keep the collagen fibrils from collapsing into a thick mat that blocks penetration of the bonding agents. A primer is more important on dentin than enamel because the primer contains hydrophilic groups that penetrate wet, etched dentin. Water is removed from etched enamel when it is dried, but the dentin remains moist (wet). For the resin to penetrate through the water, it must be dissolved in a solvent that can penetrate water and carry the resin with it. The solvents allow the resins to penetrate water on the dentin and in the dentinal tubules, and to penetrate around collagen fibrils and into porosities in the tooth surfaces created by etching. The solvents are primarily acetone, ethanol (ethyl alcohol), or a combination of ethanol and water. In general, the solvent is the largest portion of the bonding agent, making up 60% or more of the material. Acetone is a highly volatile solvent. Its rapid evaporation may require that two or more coats of the bonding resin be applied to ensure adequate sealing of the dentin. (An example of a bonding agent with acetone is Prime & Bond NT by Dentsply International, York, PA.) Ethanol evaporates more slowly, so it may need a longer drying time. (An example of a bonding agent with ethanol is Optibond Solo Plus by Kerr Corporation, Orange, CA.) All bottles of bonding agents should be recapped immediately after the material is dispensed, to prevent evaporation of the solvent, which leads to gradual thickening of the resin with less ability to penetrate etched dentin. Unit-dose (single-use) packaging of bonding agents avoids some of these problems associated with />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses