7
Obstructive Sleep Apnea
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition characterized by repetitive partial or complete upper airway obstruction during sleep. It is the result of inspiratory negative pressure overcoming the intrinsic ability of the pharyngeal soft tissues to maintain a patent airway, either through anatomical factors, hypotonic musculature, or a combination of both. This leads to episodes of hypoxia and multiple sleep arousals and subsequent sleep deprivation. As a result, OSA patients often experience reduction in quality of life, poor social function, and neurocognitive underperformance. Depression, fatigue, snoring, personality changes, and poor sexual performance are also frequent symptoms. OSA has also been associated with medical morbidity including obesity, hypertension, cardiovascular disease, stroke, metabolic syndrome, polycythemia, cor pulmonale, cardiac arrhythmias, and even early mortality. It is a highly prevalent disease affecting approximately 20% of adults using a criteria on the apnea hypopnea index (AHI) of AHI ≥5/h, with up to 10% being defined as moderate to severe disease (AHI ≥15/h).1,2 When symptoms of sleepiness are included as part of the definition, approximately 4% of men and 2% of women meet the criteria.3,4 Risk factors include obesity, male gender, advancing age, and craniofacial abnormalities such as mandibular insufficiency.
The most common and effective nonsurgical means of treatment for OSA is positive airway pressure (PAP), either continuous (CPAP) or bilevel (BiPAP). It is considered first-line treatment and works by stenting open the upper airway during sleep, thereby reducing apneic events. It has been shown to be highly effective at improving subjective measure such as quality of life and daytime sleepiness, as well as reducing cardiovascular mortality. However, long-term acceptance and adherence is low. Patients often complain of chest discomfort, dry mucous membranes, mask discomfort, congestion, and claustrophobia, among others. Ultimately, more than 50% reject CPAP therapy. Other nonsurgical methods include weight loss, oxygen therapy, pharmacological management, and a variety of oral appliances. Weight loss has been shown to be effective but is seldom sustained. Other methods have had variable results.
Given the poor adherence to CPAP therapy, many seek surgical treatment for correction of their sleep apnea. To be successful, surgery must bypass the obstructed area or prevent its collapse. Tracheostomy was the first surgical treatment for OSA, introduced by Kuhlo and colleagues in 1969.5 Ikematsu described uvulopalatopharyngoplasty (UPPP) for treatment of snoring in 1964,6 which was later adapted in the late 1970s for the treatment of sleep apnea. Kuo and colleagues first described mandibular advancement for the treatment of OSA in 1979.7 Since the early description of these surgical procedures, multiple modifications have been described and new procedures introduced. Each procedure is unique in regard to the level of obstruction it addresses, as well as its potential complications. In addition, these procedures are often combined as part of a multimodality approach, which must be considered, as this will influence the type and incidence of complications.
Full understanding of these potential complications is vital to the success of surgical treatment in the OSA patient as it facilitates not only proper management but anticipation in order to prevent their occurrence. The preoperative, intraoperative, and postoperative periods are all critical time points that can negatively affect surgical success if not managed appropriately. The goal of this chapter is to provide guidance concerning each of these time periods in regard to potential complications and their management, as well as an overview of some of the most common surgical procedures performed today for correction of OSA.
PREOPERATIVE PHASE
Medical History Considerations
Obstructive sleep apnea is associated with a multitude of comorbidities (Table 7.1). A full understanding of these associations and their management is important to the practitioner treating the OSA patient. While many of these associations are well established and have the advantage of strong clinical research that supports their relationship, others remain less clear. Regardless, when performing a thorough history, an awareness of these conditions should alert the surgeon of the possibility of their association and help direct appropriate care.
Table 7.1. Comorbidities Associated with OSA
| Obesity |
| Cognitive impairment |
| Hypertension |
| Congestive heart failure |
| Ischemic heart disease |
| Cardiac arrhythmias |
| Cerebral vascular accidents |
| Metabolic syndrome |
| Diabetes |
| Pulmonary hypertension |
| Hypothyroidism |
| GERD |
| Depression |
The initial evaluation of a patient with OSA begins with a history and physical exam, with particular emphasis on certain key elements. A thorough history is critical to the preoperative planning and prevention of surgical complications. Patients should be questioned regarding the severity of their OSA and subjective symptoms such as daytime sleepiness and level of function. Standard questionnaires filled out by the patient prior to the appointment are helpful to assess symptoms and subjective sequelae of their disease. Previous successful and unsuccessful surgical and nonsurgical treatments, including oral appliances and PAP therapy, should be reviewed. Adherence and response to PAP should also be discussed. The patient’s bed partner should be included for history of observed apneic events and snoring, and any available laboratory studies including recent sleep studies reviewed. A thorough cardiovascular and pulmonary history should be performed, and any patient with a positive history should be further questioned regarding their current level of function and about any symptoms such as shortness of breath and chest pain. Any recent studies including stress tests and echocardiograms should be requested. Vitals should be obtained and any previously undiagnosed hypertension or other finding should be dealt with prior to surgery. Any concerning findings during the patient history should be considered for further medical workup prior to surgery. This may involve the patient’s primary physician, a specialist, or the anesthesia team and will be considered in a subsequent section.
Preoperative Consultation and Medical Clearance
Patients with complex medical histories and multiple comorbid conditions should have preoperative evaluation by a primary care physician or appropriate specialist prior to surgery. Hypertension should be adequately controlled before any surgical procedure. Hypertension is often undiagnosed in OSA patients, and it is therefore important to screen patients on evaluation. Poorly controlled blood pressure preoperatively will make hypertension more difficult to manage in the perioperative period. It should be noted that many of these patients can have difficult to treat or treatment-resistant hypertension.8 Patients with diabetes should have optimization of glucose control prior to surgery. The patient’s primary care physician or cardiologist should assess the patient’s level of function and provide clearance for surgery in patients with known cardiovascular disease. The patient should be educated about the risks of surgery with full disclosure about how their medical conditions may affect outcome. Any other information concerning medical comorbidities should be managed prior to surgery to optimize outcomes and reduce the risk of surgical complications.
Patients with OSA should also have a preoperative evaluation by an anesthesiologist. Open communication between the anesthesia and surgical teams facilitates appropriate treatment planning. While OSA patients generally are assumed to have more difficult airways, those who present with particularly challenging airways may require a more well-developed plan. Indicators of difficult ventilation and intubation revealed in the physical exam must be communicated and contingency plans discussed. The anesthesiologist should be made aware if more invasive techniques are anticipated such as elective tracheostomy.
Anesthesia Considerations
The next decision involves the selection of the type of anesthesia to be used. Whenever possible, surgical procedures should be performed with local or regional anesthesia. Care should be exercised when conscious sedation is employed in this patient population. American Society of Anesthesiology (ASA) status and comorbid conditions only represent part of the problem when discussing anesthetic risk in OSA patients. Their narrow and easily collapsible airways may be compounded by anesthetic-induced relaxation on the pharyngeal musculature. Surgery on the airway results in varying levels of edema and resultant narrowing. Sleep deprivation secondary to their disease as well as anxiety about the upcoming procedure may result in accidental oversedation. Additionally, if airway obstruction does occur, it is not uncommon to encounter difficulty with ventilation and intubation in these patients. Conscious sedation, therefore, should be used sparingly and only in select nonsevere cases under supervision of a practitioner experienced in airway management. Sedatives should be titrated slowly to effect to avoid oversedation. Deep sedation is rarely indicated due to the risk of airway loss. General anesthesia with a secure airway is preferred over moderate or deep sedation, even in closely monitored situations such as in the operating room.
Preoperative Medications
It is common practice to provide sedatives, anxiolytics, or narcotics prior to transport to the operating room in many surgical centers. These medications serve to alleviate patient anxiety and improve the transition to the operating suite. While safe in most instances, it should be practiced with caution in OSA patients. While the most common agents, benzodiazepines, depress respiratory drive less than most narcotics, the potential for ventilatory compromise in these patients may have serious consequences. Death in the preoperative area has been reported.9 This practice may be used in certain centers with appropriately trained personnel and well-established protocols including one-on-one supervision and continuous vital sign monitoring. In the absence of these provisions, it is generally best to avoid preoperative sedatives in OSA patients.
Obesity, GERD, and the potential for difficult, prolonged intubation may increase the risk of aspiration in OSA patients. The use of a combination of proton pump inhibitor, H2 blocker, antacid, or esophageal motility stimulant has been advocated to help prevent aspiration on induction. These patients are also at increased risk upon extubation and gastric suctioning is advised at the end of the procedure.
Preoperative CPAP
As mentioned previously, patients are often sleep deprived prior to surgery. This sleep deprivation is a result of OSA-related extreme daytime sleepiness (EDS) and anxiety about the upcoming procedure. After surgery and general anesthesia, the patient is more likely to enter deep sleep and may be predisposed to more severe sleep apnea.10 The goal of CPAP therapy prior to surgery is to limit the accumulation of sleep debt and reduce deep sleep rebound in the perioperative period. Some recommend the CPAP be worn for 2 weeks prior to any planned surgical procedure.11 One potential hurdle is that patients who are undergoing surgery for OSA are often CPAP noncompliant. Even modest use before surgery may be beneficial, however, and should be encouraged.12
INTRAOPERATIVE PHASE
Airway Management
OSA patients are generally believed to produce more challenging airways and may present difficulty in ventilation and intubation. OSA patients are often obese, male, have increased neck circumference, higher Mallampati scores, and other craniofacial abnormalities that may be associated with a more difficult airway. They may also have excessive, floppy oral and pharyngeal airway tissues, or significant obstructions anywhere along the upper airway. The patients should be evaluated by an anesthesia provider preoperatively and communication with the surgical team should be provided to reduce the incidence of postoperative complications through proper planning. Important physical exam findings that may predict difficult intubation include mouth opening, protrusion of teeth, Mallampati scores, jaw protrusion, mandibular length, neck circumference, and neck length and mobility.
Excess saliva production and aspiration risk should be reduced preoperatively with an antireflux agent and antisialagogue.13 Before induction, a 3- to 5-minute period of 100% oxygen administration should be employed to maximize oxygen reserves in preparation for a potentially prolonged intubation. The risk of inability to ventilate should always be anticipated, and preoxygenation allows for increased working time for airway establishment. Induction should be accomplished with short-acting intravenous anesthetic such as propofol. Additionally, if a neuromuscular blockade is used, preference should be given to an agent with quick onset and short duration of action, such as succinylcholine. The use of short-acting agents is important in case ventilation and intubation are unsuccessful. Ventilation success begins with proper positioning and preparation for a difficult airway. The patient should be positioned with a head-tilt, chin-lift, or jaw-thrust technique to open the airway. Reverse Trendelenberg position may be used to relieve intrathoracic pressure and improve ventilation. An oropharyngeal or nasopharyngeal airway of appropriate length to extend past the hypopharyngeal obstruction should be used. A two-person ventilation technique may be required in order to provide adequate mask seal and jaw positioning. A laryngeal mask airway (LMA) can also be placed for ventilation and used later to intubate through.14 LMA allows ventilation while overcoming the soft tissue obstruction and provides a portal for suctioning and intubation. Patients that are unable to be ventilated using these techniques may require more advanced procedures such as percutaneous transtracheal jet ventilation or cricothyrotomy until a definitive airway can be established.
While intubation may be accomplished in a standard fashion with direct laryngoscopy, alternative methods are available and are frequently utilized (Table 7.2). The selection of one particular method often depends on preoperative exam findings and the anticipation of a difficult airway. Options for intubation include awake oral or nasal, awake or asleep fiberoptic, and intubation through an LMA. Newer techniques include utilization of a light wand or video laryngoscopy and are growing in popularity. Awake procedures may be preferred in the incidence of preoperative exam findings consistent with difficult intubation. Boyce and colleagues described the use of transnasal jet ventilation-assisted fiberoptic intubation. In this technique, a transnasal jet is used through a nasopharyngeal airway to provide intermittent flow of pressurized air to the upper airway. The purported advantages are improved visualization and simultaneous ventilation of the patient during fiberoptic intubation. They reported no incidences of serious complications and high intubation success rates.15,16 In their prospective study of 180 morbidly obese patients undergoing bariatric surgery, Neligan and colleagues used direct laryngoscopy after standard induction with the patient in the “ramped” position, with the head and shoulders above the chest. The incidence of OSA in this population was 68%. They found this technique to be very successful and had no incidences of rescue airway use. The difficult intubation rate was only 3.3% (defined as three or more intubation attempts). Interestingly, they found no relationship between body mass index (BMI), neck circumference, or presence of OSA and the difficulty of intubation. They did note male gender and higher Mallampati scores to be predictive, however.17 Finally, in select cases of patients with severe disease and significant medical comorbidity, such as life-threatening arrhythmia, and failed intubation attempts, an elective tracheostomy may be considered. The surgeon may also be required to perform a tracheostomy or cricothyrotomy emergently if the patient cannot be ventilated.
Table 7.2. Techniques for Difficult Intubation
| Awake oral or nasal traditional intubation |
| Blind nasal intubation |
| Intubating LMA |
| Awake or asleep fiberoptic intubation |
| Retrograde intubation |
| Light wand technique |
| Video laryngoscope |
| Transnasal jet ventilation assisted fiberoptic intubation |
Extubation is best performed in the operating room in a controlled setting. The patient should be extubated awake, once spontaneous ventilation, the ability to follow commands, and ability to sustain a head lift have returned. Full reversal of neuromuscular blockade should be verified. In patients who were easy to ventilate prior to intubation whose surgery did not affect the airway, deep extubation may be considered. It is advised to allow the patient to awake sufficiently as is needed to protect their airway and avoid suspending extubation until the patient is in the recovery room or ICU. This practice will hopefully minimize complications secondary to airway compromise.
Blood Pressure Control
Hypotensive anesthesia, particularly during maxillary osteotomies, may be used to help control blood loss. This may be difficult to achieve intraoperatively if adequate control has not been obtained prior to the procedure. This underscores the importance of appropriate management and consultation preoperatively. The need for hypotensive anesthesia, however, must be weighed against the risk of inadequate organ perfusion. In patients at risk for ischemic heart disease or stroke, hypotensive anesthesia may need to be abandoned to reduce surgical events.
The proper application of the technique involves communication between the anesthesia and surgical team. The anesthesia team must be given adequate time prior to the osteotomy to safely lower the blood pressure in a slow, controlled fashion to minimize the risk of an unsafe drop and inadequate perfusion. In addition, blood pressure should be allowed to return to normal as soon as possible. This will allow minimum time under hypotensive anesthesia and hopefully reduce cardiovascular or neurologic complications. In patients felt to be inadequate to withstand anesthesia-induced hypotension, preparations should be made and the patient educated about the possibility of blood transfusion.
Additional Considerations
Proper patient positioning and adequate padding are important to reduce pressure ischemia. Obesity, long procedures, and hypotensive anesthesia can all potentially exacerbate its occurrence and must be taken into consideration. Both steroids and the judicious use of intravenous fluids intraoperatively may help reduce the risk of airway edema and respiratory compromise. Additionally, narcotics should be used sparingly whenever possible in this patient population during the procedure. When needed, it is better to use shorter-acting agents. Postoperatively, the cumulative effect of excessive narcotic administration may contribute to respiratory compromise.
SURGICAL PROCEDURES
After diagnostic evaluation of the OSA patient and identification of the level of obstruction, a surgical procedure must be selected. Surgical treatment of OSA involves a variety of procedures that address the obstruction at multiple levels. Properly matching the type of obstruction to the procedure is paramount to success. The nasal cavity, nasopharynx, oropharynx, and hypopharyx are all potential sites of obstruction that may contribute to the pathogenesis of OSA. Surgical procedures for OSA can be classified by the types of structures being modified (soft tissue, hard tissue, or both) or by anatomical location (nasal airway, nasopharynx, oropharynx, hypopharynx, or a combination) (Table 7.3). As mentioned previously, there have been many procedures described for the treatment of OSA. It is important that any practitioner performing these procedures be well educated on the potential complications. For those procedures not described here, they have been extensively covered in other texts. The following is a review of the most common procedures used to treat OSA by anatomic location, and a discussion of associated complications and their management.
Table 7.3. Location of Upper Airway Obstruction and Common Surgical Procedures
| Anatomic Location of Obstruction | Tissue Type | Procedure |
| Nasal | Soft | Turbinectomy, polypectomy |
| Hard | Septoplasty, alar base reconstruction, nasal valve reconstruction | |
| Oropharyngeal | Soft | Tonsillectomy, UPPP, LAUP, radio-frequency palate tissue reduction (somnoplasty), Pillar palatal implants, transpalatal advancement, pharyngoplasty |
| Hard/soft | Maxillary expansion, maxillary advancement | |
| Hypopharyngeal | Soft | Glossectomy, radio-frequency tongue base ablation, mandibular distraction |
| Hard/soft | Genioglossus advancement, hyoid advancement | |
| Oropharyngeal and hypopharyngeal | Hard/soft | MMA |
| Bypass procedures | Tracheostomy |
Nasal Cavity
The role of nasal obstruction in OSA is different from that which occurs at other levels. It does not cause the primary obstruction central to the pathology of sleep apnea, but may contribute to the disease process. Increased resistance to nasal airflow can lead to turbulent flow and increase the incidence of mouth breathing. Oral breathing alters upper airway dynamics and can predispose to obstruction. Obstruction can occur by a variety of mechanisms including septal deviation, turbinate hypertrophy, polyps, and valve collapse.
Surgical procedures directed at the cause of the obstruction include septoplasty, turbinate reduction, polypectomy, and valve reconstruction. They are generally not curative but may improve AHI and CPAP pressure requirements. They have also been linked to improvement in subjective outcomes such as daytime energy levels.18 It is often performed as part of a multimodality approach as opposed to a stand-alone procedure for the treatment of sleep apnea.
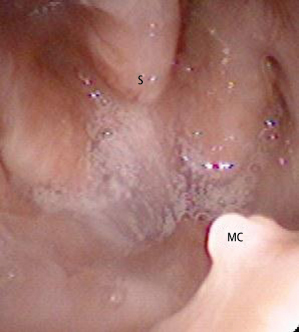
Deviation of the nasal septum may cause obstruction along any portion, and it is important to identify the area responsible prior to surgery. High deflections may mimic a weak or collapsed internal nasal valve and should be treated appropriately.19 Septal hematoma is a potential complication and can be minimized with the use of septal splints for 1 week or mattress sutures. Mattress suturing through the mucosa and septum is accomplished with 4-0 chromic gut suture on a straight needle. Mucosal tears may be observed if they occur on one side of the mucosa. For through-and-through tears, closure of at least one side with 5-0 chromic gut should be completed. Often, septoplasty is performed simultaneously with maxillary advancement. The amount of removal varies and may range from minor trimming when the septum is straight but subject to deflection with movement of the maxilla, to formal septoplasty when concomitant septal deviation contributes to the obstruction. During postoperative nasopharyngolaryngoscopy, it is common to encounter a defect in the posterior portion of the septum after maxillomandibular advancement (MMA) with combined septoplasty (Fig. 7.1). It should be noted that this is often of little clinical consequence, and should require no intervention. As long as an anterior septal strut is maintained, changes in the nasal morphology such as nasal tip deprojection should not occur.
Fig. 7.1. Septal defects after MMA. Perforations of the posterior septum are incidental findings by nasopharyngoscopy. Septoplasty combined with Le Fort I osteotomy often causes asymptomatic septal defects. S: septum, MC: maxillary crest.
Turbinate hypertrophy may be addressed with a variety of techniques. Submucosal radiofrequency and electrocautery turbinate reduction, laser turbinate excision, partial or total inferior turbinate removal, turbinate outfracture, and submocosal turbinoplasty are all frequently employed methods. Postoperative bleeding, tissue edema and subsequent worsening of obstructive symptoms, atrophic rhinitis, and nasal crusting are all potential complications. Rhinitis sicca (excessive dryness) may occur and lead to additional crusting and bleeding. It should be noted that opening of the nasal passages may lead to worsening of this condition if presented preoperatively. The use of submucosal techniques may decrease crusting and maintain ciliary function. Radiofrequency submucosal reduction has the advantage of producing little edema and does not generally require nasal packing. It takes 8 weeks to produce maximum effect, however, and multiple procedures may be required to achieve optimal result. For any nasal surgery, nasal packing should generally be avoided, as obstruction can worsen in the postoperative period. Nasal stents or tubes that allow patency of the airway are.19
Oropharynx
Tonsillectomy
Adenotonsillectomy is the procedure of choice for OSA in children, and tonsillectomy may be useful in the treatment of adult patients with enlarged tonsils and without other major airway abnormalities.20 A meta-analysis of pediatric patients with uncomplicated disease revealed a reduction in AHI of 13.9 events per hour and normalization of AHI in 80% of patients.21 Outcomes are affected by upper airway anatomy, nasal inflammatory disease, obesity, and general medical condition. Pain, swelling, and bleeding are the most common complications. Electrocautery and ultrasonic blades have been used to reduce bleeding but are still associated with postoperative pain. Other methods include the use of serial reduction with a carbon-dioxide laser in the outpatient setting aimed at reducing postoperative pain and bleeding, though this has been associated with tissue regrowth and recurrent infection.22 Radiofrequency ablation may be performed in a single setting or serially and has been associated with decreased pain, but some have expressed concern with increased chance of hemorrhage. Life-threatening complications are rare, but postoperative respiratory failure requiring mechanical ventilation can occur in up to 30% of children.23,24
Palatal Procedures
UPPP is a common surgical procedure designed to reduce obstruction by selective removal of pharyngeal tissue to enlarge the oropharyngeal airway lumen. It was first described as a technique to treat snoring,6 and later adapted by Fujita and colleagues for the correction of sleep apnea.25 Generally it involves the removal of redundant tissues of the soft palate, tonsils, tonsillar pillars, and uvula. Since it was initially described, many technical variations have been proposed. Despite variations in technique, the ultimate goal is to widen the posterior airway space. Despite reports of low success rates of 40–60% and cure rate (defined as an AHI of <5%) of 16% in several meta-analyses, it remains the most widely performed OSA pharyngeal surgical procedure today.26 Evaluation of candidates for the procedure has evolved, and thus development of more sophisticated methods has led to improved results. The Friedman staging system of the oropharyngeal airway is based on the tonsil size, a modified Mallampati classification, presence or absence of severe obesity, and craniofacial abnormalities. It helps identify patients at risk for sleep apnea who present with snoring symptoms and also has positive and negative predictive values for UPPP. Higher success rates for Friedman stage I have been demonstrated over stage III for palatal surgery alone. Performance of UPPP blindly without proper identification of the level of obstruction has likely contributed, historically, to poor success.
Complications are common and include bleeding, severe pain, dysphagia, pharyngeal discomfort, mucosal dryness, wound infections, and taste and speech disturbances. Dysphagia can be persistent in up to 30%, though it is generally mild. Major complications are rare and include velopharyngeal insufficiency, acute respiratory distress, nasopharyngeal stenosis, and hemorrhage. As previously discussed, early reports indicated high frequency complications associated with UPPP, including serious events such as respiratory compromise and death.9,27 More recent reports, however demonstrate a trend towards lower rates.28 One systematic review reported serious complications with an incidence of 2.5% (with 0.2% mortality). However, persistent side effects were high at 58% (31% nasal regurgitation, 13% voice changes, and 5% taste disturbances).29 Complications such as dysphagia, discomfort, and pain may be difficult to avoid. Management generally involves proper patient education and expectant management. Care should be taken in administration of postoperative narcotics and strategies to minimize their use. This is particularly important after upper airway procedures where the potential for respiratory compromise exists secondary to pharyngeal edema. The use of steroids, tissue coolants, and blood pressure control also may decrease edema and will be discussed later. The patient should also be properly informed about the risk of failure and possibility for additional procedures. The majority of avoidable complications, however, are due to aggressive tissue removal. Management generally involves judicious excision followed by meticulous tissue rearrangement and closure.30 Additionally, nasal CPAP tolerance may be decreased due to mouth leak from excessive tissue removal, further justifying the need for conservative surgery.
Generally performed in the office under local anesthesia, laser-assisted uvulopalatoplasty (LAUP) involves the partial removal of the palate and uvula via laser. Usually indicated for the treatment of snoring, its utilization for surgical management of sleep apnea is controversial. Two randomized clinical trials found no change in the AHI after surgery when used as a treatment for OSA.31,32 Additionally, other concerns include studies that have suggested no statistical significance in daytime sleepiness, and possible worsening of OSA after undergoing the procedure.26 Complications are similar to those for UPPP, and include severe pain, bleeding, infection, globus sensation, velopharyngeal insufficiency (VPI), and taste changes. Globus sensation may require a secondary procedure to break up scar banding. Staged or “titrated” surgeries with reevaluation prior to each step reduce occurrence of VPI.20
Hypopharynx
Soft tissue procedures
Glossectomy
Designed to eliminate hypopharyngeal obstruction, midline glossectomy and linguoplasty are partial glossectomies performed usually via laser. Surgical success rates have been reported from 25% to 83%, with an average of approximately 50%.33 Complications approach 25% and include bleeding, severe odynophagia, tongue edema, and taste changes. Due to morbidity and poor success of the procedure, glossectomy has been relegated to a limited population of patients who are not candidates for other procedures. Additionally, historically perioperative tracheostomies were often performed due to risk of postoperative airway obstruction resulting in high morbidity.20
Radiofrequency Ablation of the Tongue Base
Radiofrequency ablation of the tongue base is often performed in the office setting under local anesthesia. It may be either performed as a single-modality treatment or often in conjunction with other airway procedures. Randomized controlled studies have demonstrated effectiveness of the procedure in reducing OSA severity and improving quality of life. This has also been demonstrated in the long term. Complications include abscess, tongue base weakness, alteration of speech and swallowing, cellulitis, and airway obstruction and edema. Fortunately, these complications are rare.20 Mucosal ulcerations can be avoided by not placing the electrodes too superficially. Abscesses and infections can be reduced with preoperative antibiotics, but may require surgical drainage. The middle third of the tongue s/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


