Chapter 7
Injectable Fillers for Cosmetic Facial Enhancement
Introduction
With increasing age and sun exposure, changes occur at all tissue levels in the face to effect “aging.” The skin thins, primarily as a result of thinning of the dermis. It also becomes less elastic secondary to its loss of organization of elastic fibers and thinning. There is a loss of subcutaneous fat in selected areas and an accompanying redistribution of facial fat. There are not significant changes in the voluntary muscles; however, there is modeling and remodeling of the facial bones resulting in less fullness, especially in the infraorbital and piriform rim areas.
Conjointly these changes result in facial features consistent with less volume and laxity and wrinkles. For these reasons, injectable facial fillers can have a very positive effect on the aging face.
Injectable fillers for cosmetic facial enhancement are the second most commonly requested service in facial cosmetic surgery practices. Although various fillers are espoused by different individuals, and virtually all fillers are promoted by their manufacturers as good for all areas of the face, this chapter will discuss only the currently U.S. Food and Drug Administration (FDA)-approved facial fillers and discuss each individual filler in the context of how the author has found it most useful, specifically in which anatomical areas.
FDA approval of the injectable fillers is explicitly for specific use. However, off-label use is permitted and legal. In these instances, patient acceptance and understanding are to be included in the informed consent. It is not legal to either purchase or administer non-FDA-approved injectable fillers.
The following FDA-approved injectable fillers will be sequentially discussed: collagen; hyaluronic acid; calcium hydroxyapatite (Radiesse, Bioform Medical Inc., San Mateo, CA); poly-L-lactic acid (PLLA) (Sculptra, Adventis Pharmaceuticals, Bridgewater, NJ); and polymethylmethacrylate (PMM) and bovine collage (Artefill, Suneva Medical Inc., San Diego, CA).
During the last several decades, augmentation of various aspects of the face have been practiced and improved with various autogenous, allogenic, and artificial materials. With the development of injectable fillers over the last two decades and their FDA approval, considerable additional improvements and advances have occurred. This, coupled with an increased interest and public knowledge, has not only significantly increased their use in cosmetic facial surgery practices but also resulted in some confusion regarding each product’s optimal utility. This chapter will present an evidence-based approach for the use of available FDA-approved injectable fillers and will discuss the specifics for each filler including the optimal anatomical injection locations, duration of effectiveness, and possible complications.
Injectable fillers offer an excellent modality of treatment for a number of inherent and age-related facial volume deficiency concerns. These vary from the traditional lip augmentation to nasal labial folds to marionette grooves to tear troughs to panfacial augmentation. The author will discuss his preferred fillers for each of these anatomical areas.
Finally, four of the discussed fillers have all been tested and initially FDA-approved for use in the nasal labial fold model, with Sculptra being the exception. Therefore, most uses in clinical practice and as described herein are technically off-label.
Collagen (bovine and human)
Bovine collagen products (Zyderm-1, Zyderm-2, and Zyplast from Inamed Corp., Santa Barbara, CA) were the first FDA-approved injectable fillers. Since they were derived from bovine collagen, skin testing was required. For many years, these were the only injectable fillers and worked reasonably well. Indeed, they dominated the market for almost two decades. However, their longevity, although in part related to how carefully they were injected into the dermis, was generally short, about 3 months. Currently, these collagen products are no longer available.
However, in the last decade, a number of additional bovine and human collagen injectable fillers have been introduced and FDA-approved. In 2003, Inamed Corp. introduced CosmoPlast and CosmoDerm, human collagen injectable fillers derived from cultured human infant foreskin. The latter is noncross-linked, whereas the former is cross-linked. CosmoDerm is indicated for fine wrinkles, whereas CosmoPlast is recommended for deeper wrinkles. No allergy skin testing is necessary.
Unfortunately, their longevity has not been proven to exceed that of their bovine collagen predecessors. Additional collagen-based products have and will likely continue to be introduced; however, currently, the injectable fillers to be subsequently discussed offer advantages in terms of duration of effect. All of the subsequently discussed fillers have been studied with respect to their longevity relative to collagen (Zyplast) and have been documented to last longer.
Hyaluronic acid fillers
Hyaluronic acid is naturally more concentrated in the skin than anywhere in the body. Due to its hydrophilic and viscoelastic properties, it keeps the skin from appearing dry and wrinkled.
In late 2003, Restylane (Q-Med, Uppsala, Sweden) was the first hyaluronic acid filler FDA-approved in the United States. This filler was met with considerable public enthusiasm, in part due to the fact that it did not require skin testing since hyaluronic acid is chemically, physically, and biologically similar in all species. In addition, this filler was proven to last much longer than collagen. Subsequent introduction and approval of Perlane, Restylane Fine Line, and Restylane SubQ occurred from the same company.
Since the introduction of Restylane, several additional hyaluronic acid fillers have been FDA-approved, including Hylaform (Inamed), Captique (Inamed), and Juvéderm (Allergan Inc., Irvine, CA). Juvéderm has two products, Juvéderm Ultra (24 HV) and Juvéderm Ultra Plus (30 HV). The latter is more highly cross-linked and recommended for deeper folds and wrinkles.
Basic biology
The author prefers Juvéderm due to its flow properties, duration of action, and improved patient acceptance. Detailed clinical studies regarding the longevity of hyaluronic acid have been conducted in humans, in their nasal labial folds, and both compared with bovine collagen (Zyderm) and evaluated independently with respect to absolute duration. All available studied hyaluronic acid injectable fillers had significantly longer duration of action than collagen. Individual longevity studies of the various hyaluronic acid fillers indicate that they persist about 12 months.
Isovolemic degradation accounts in large part for the longevity of injected hyaluronic acid. This results in hydrophilia, water being drawn into the filler to the tune of it binding to 1000 times the actually injected fillers’ weight in water. As the filler degrades, water is continually drawn into the filler molecules. Hyaluronic acid products are removed from the body by endocytosis, with cellular breakdown at the site and lymphatic uptake, partial depolarization, and exchange into the circulation.
Indications
The author uses hyaluronic acid injectable fillers exclusively for lip augmentation and also as an option for tear trough correction. They also can be used for many other areas. However, the author believes that the other injectable fillers offer advantages in these other areas as will subsequently be discussed.
The injection of Juvéderm varies somewhat with the product, Ultra versus Ultra-Plus, but is essentially ideally injected into the mid to deep dermis, as are all of the hyaluronic acid injectable fillers. All hyaluronic acid fillers are supplied in preloaded 0.8-mL syringes, and Restylane also is available in partial syringes of 0.4 mL. They are injected with 27- or 30-gauge needles, 1/2 in. in length. They are stored at room temperature. If possible, 7–10 days prior to the injection, the patient is asked to discontinue the most commonly taken over-the-counter medications that accentuate bleeding and therefore bruising. These include aspirin (ASA), nonsteroidal anti-inflammatory medications, high doses of vitamin E, and several herbal preparations including St. John’s wort, alfalfa, feverfew, ginkgo, as well as others.
Technique
It is recommended that the filler not be injected so as to overcorrect. Indeed, the author makes it clear to patients that due to the long-lasting nature of these products, overcorrection is inadvisable, and in 2–3 weeks additional product can be added if desired. For treatment of areas other than the lips, no local anesthesia is employed. A small ice pack is applied for 30–60 seconds by the patient prior to injection. For lip augmentation, pain control is important. This has been achieved in a variety of fashions, including local anesthesia blocks, infiltration anesthesia, topical anesthesia, and light sedation. The author has employed all of these methods and has found that the use of a topical anesthetic composed of 7% lidocaine and 7% tetracaine in a cream base when applied for 30 minutes prior to injection, with or without inhalation of 50/50 nitrous oxide/oxygen, is the most patient-accepted. The author has this mixture fabricated by a local compounding pharmacy.
There are three basic injection techniques for facial fillers: linear threading, serial puncture, and cross-hatching. In the lips, the author uses the linear threading method, although some prefer serial punctures. Each has its application in certain circumstances and will be commented on throughout this chapter. Prior to injection, the areas to be treated are cleansed with alcohol or antiseptic soap. Also, immediately prior to injection, 0.1–0.2 cc of 2% lidocaine with 1,100,000 epinephrine is mixed into the Juvéderm by use of a Baxa RapidFill™ Connector (Baxa Corp., Englewood, CO) that simultaneously allows Luer lock attachment of two syringes to mix. This technique reduces discomfort and bruising.
Figure 7.1 illustrates the author’s most commonly utilized lip augmentation approach. When the needle is inserted into the white roll of the lip and advanced in the proper tissue plane, it appears as if a tunnel exists in this plane and as the Juvéderm injection begins with slow withdrawal, one can visualize the filler advancing beyond the needle tip in this plane; that is, beyond the distance that the needle was inserted. Generally, one syringe is sufficient to perform a “Paris Lips” correction. This procedure is intended to accentuate the definition of the lips, reduce some wrinkles that bridge cutaneous-mucosal tissues, and effect sight size increase.
Figures 7.1 and 7.2 Paris Lips injection technique (*illustrations).
(From Epker BN. Esthetic Maxillofacial Surgery. Philadelphia: Lea and Febinger, 1994.)
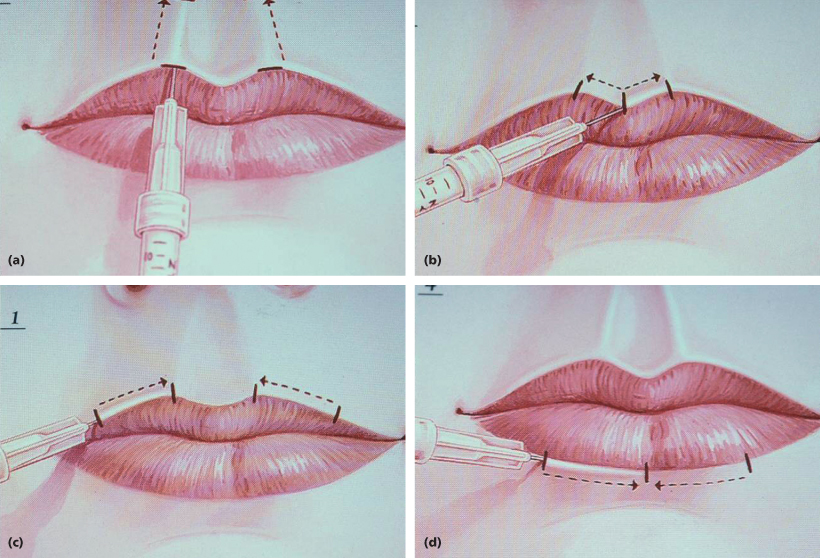
Additionally, these products can be used for absolute volume increase in the lips by injection of the material at about the wet line in one or both lips. Recalling that ideally the lower lip vermillion is about one-third fuller, more product is generally placed in the lips so as to retain or achieve this relationship. In certain individuals, both volume enhancement and the Paris Lips procedure are performed simultaneously.
Once the injections are performed, the material is either gently compressed or lightly massaged between the thumb and a finger, or a cotton-tip applicator can be firmly rolled over the area to make certain it is evenly distributed.
Untoward sequelae
Following the injection of hyaluronic acid fillers, the area will become slightly swollen, red, and tender. This generally resolves within 24–48 hours. Minor bruising can occur but is not common. It is helpful to apply ice to the areas for a brief period, both prior to and following the injection. Initially, the injected area is slightly firm, but this rapidly resolves within the first week. If lumpiness exists, this can be resolved with gentle massage, such as between two fingers.
Patients are scheduled for a follow up in 7–10 days to check the result and are instructed to call if swelling or bruising occurs and seems excessive. They are informed that most swelling should subside within 24 hours. No restrictions or limitations on their physical activities are placed on patients following treatment.
Results of treatment
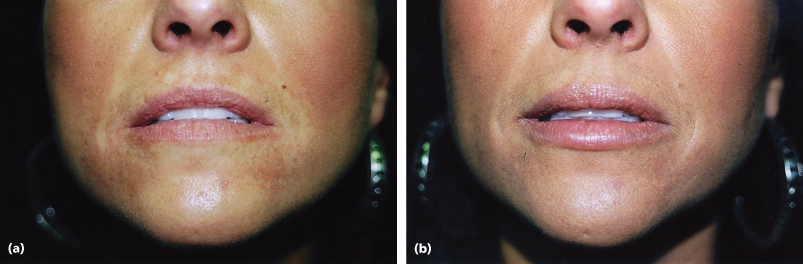
Results of treatment are seen in Figures 7.2 and 7.3 for both the classic Paris Lip augmentation and combined Paris Lips and absolute lip volume enhancement.
Figure 7.3 (a and b) Paris Lips procedure to enhance a natural-looking appearance with increased overall definition and volume.
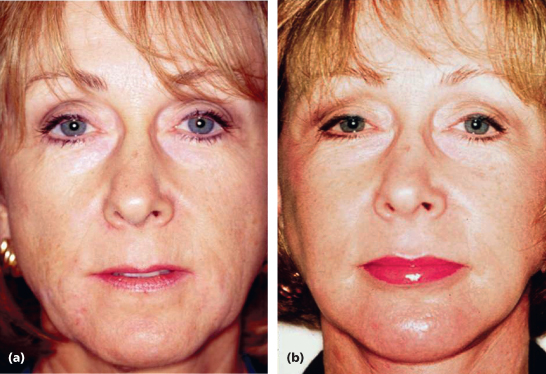
Calcium hydroxyapatite (Radiesse)
Oral and maxillofacial surgeons have used hydroxyapatite for almost 25 years to fill bony defects and to contour virtually all aspects of the craniofacial skeleton. In 2006, an injectable calcium hydroxyapatite (Radiesse) was FDA-approved for soft tissue correction. Radiesse consists of uniform calcium hydroxyapatite microspheres, ranging in size between 25 and 45 µm in diameter, and aqueous carboxyl and methylcellulose. These particles constitute roughly 30% of the product and are suspended in the aqueous carboxyl methylcellulose gel. This product is currently FDA-approved for correction of deep wrinkles, nasolabial folds, and HIV facial lipodystrophy.
Basic biology
Longevity studies have shown the persistence of calcium hydroxyapatite in the vocal folds, where it is used to correct vocal cord paralysis, for up to 8 years, and longitudinal studies of nasolabial folds have shown it to substantially outlive Zyderm. Indeed, studies at 24 months following nasolabial fold correction have reported a 70% patient satisfaction. Most studies and experiences have revealed that Radiesse is both effective and safe, displaying these characteristics for a period of time greater than that of the hyaluronic acid fillers for the nasolabial folds, marionette grooves, noses, malar eminences, and in patients with HIV lipodystrophy.
Following injection of Radiesse into the deep dermis or dermal subcutaneous area, the gel carrier is reabsorbed over several months and replaced with a fibrovascular collagen-like stroma. As a result of detailed histological and immunohistochemical analysis, it has been determined that t/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


