General Pathology
Inflammation2
A A host response to cellular injury that consists of vascular responses, migration and activation of leukocytes, and systemic reactions
B Cellular injury may occur because of trauma, genetic defects, physical and chemical agents, tissue necrosis, foreign bodies, immune reactions, and infections
C A protective response designed to rid the body of the initial cause of cell injury and the consequences of that injury
D Inflammatory response consists of a vascular reaction and a cellular reaction
1. Reactions are mediated by chemical factors derived from plasma proteins or cells
2. Reactions are produced in response to or activated by the inflammatory stimulus
E Cardinal signs of inflammation
1. Rubor (redness)—caused by increased vascularity
2. Tumor (swelling)—caused by exudation of fluid
3. Calor (heat)—caused by a combination of increased blood flow and the release of inflammatory mediators
4. Dolar (pain)—caused by the stretching of pain receptors and nerves by inflammatory exudates and by the release of chemical mediators
5. Functio laesa (loss of function)—caused by a combination of the above effects
G Cells involved in inflammation (Figure 7-1)
1. Neutrophil or polymorphonuclear leukocyte (PMN)
a. Second white blood cell to emigrate to injured tissue, where it becomes a macrophage
b. Capable of phagocytosis; helper during the immune response
Acute Inflammation
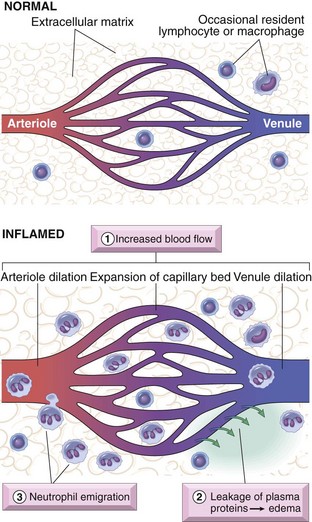
A Vascular changes (Figure 7-2)
1. Transient vasoconstriction of arterioles lasting several seconds
a. First involves arterioles, and then results in the opening of new capillary beds
b. Results in increased blood flow, which causes heat and redness
c. Induced by chemical mediators such as histamine and nitric oxide on vascular smooth muscle
3. Increased permeability of microvasculature
a. An exudate of protein-rich fluid escapes into extravascular tissue, resulting in edema
b. Proposed mechanisms that explain the leakage of endothelium in inflammation to allow this response are:
(1) Formation of endothelial gaps in venules elicited by chemical mediators
(2) Direct endothelial injury resulting in endothelial cell necrosis and detachment
(a) Begins after a delay of 2 to 12 hours
(b) Lasts for several hours or days
(c) Involves venules and capillaries
(d) Caused by thermal injury, radiation, and certain bacterial toxins
(4) Leukocyte-mediated endothelial injury
(5) Increased transcytosis across the endothelial cytoplasm
(6) Leakage from new blood vessels until new endothelial cells mature and form intercellular junctions
4. Concentration of red cells in small vessels and increased viscosity of blood, known as stasis
5. Leukocytes, mainly neutrophils, accumulate along the vascular endothelium (margination); the endothelium becomes lined by leukocytes (pavementing); leukocytes adhere to the endothelium and, soon after, migrate through the vascular wall into interstitial tissue (diapedesis or emigration)
1. Chemical attraction of leukocytes to emigrate in tissues
a. Mannose receptors and scavenger receptors bind and ingest microbes
b. Phagocytosis is greatly enhanced by opsonins, specific proteins such as immunoglobulin G (IgG) antibodies, fragments of the complement protein C3, and plasma lectins that are recognized by specific receptors on leukocytes
a. Extensions of the cytoplasm flow around the particle to be engulfed, resulting in the complete enclosure of the particle within a phagosome created by cell’s plasma membrane
b. Neutrophils and monocytes become degranulated during this process
a. Eliminate infectious agents and necrotic cells
b. After the killing, acid hydrolases degrade the microbes within phagolysosomes
4. Release of leukocyte products
a. Include lysosomal enzymes, reactive oxygen intermediates, and products of arachidonic acids such as prostaglandins and leukotrienes
b. These products may cause endothelial injury and tissue damage
c. If unchecked, this leukocyte infiltrate becomes harmful and is associated with many chronic systemic diseases
d. Also produce growth factors that aid in repair after tissue injury
5. Apoptosis-defined as programmed cell death used by the body to eliminate old cells
D Chemical mediators (Table 7-1)
TABLE 7-1
Actions of the Principal Mediators of Inflammation
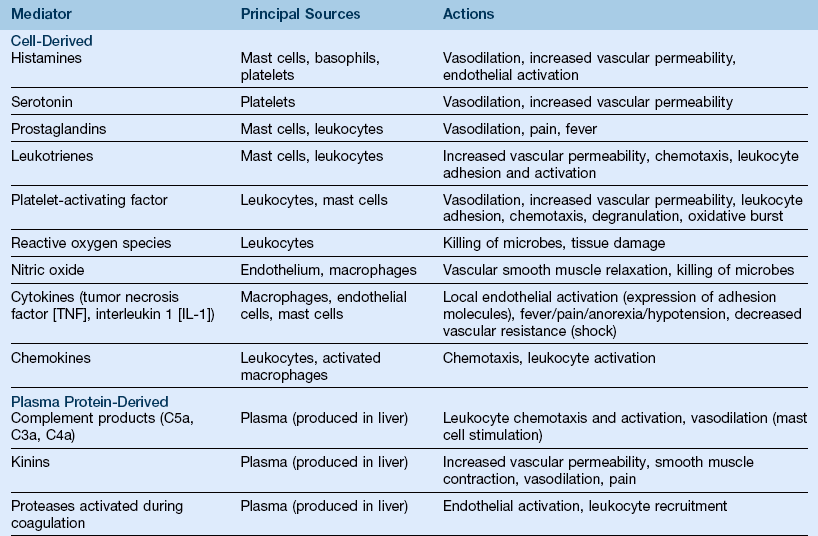
(From Kumar V, Abbas AK, Fausto N Aster JC: Robbins and Cotran pathologic basis of disease, ed 8, Philadelphia, 2010, Saunders.)
a. Originate from plasma proteins or from cells
(1) Plasma-derived mediators such as complement proteins and kinin are in precursor form and must be activated
(2) Cell-derived mediators such as histamine, prostaglandins, and cytokines come from mast cells, platelets, neutrophils, and monocytes or macrophages
b. Production of active mediators is triggered by microbial products or by host proteins
c. Mediators perform their activities by binding to specific receptors on target cells, having direct enzymatic activity, or mediating oxidative damage
d. Once activated, most mediators are short lived, but cause harmful effects
(1) Consists of 20 component proteins found in greatest concentration in plasma
(2) Causes increased vascular permeability, chemotaxis, and opsonization (the process by which certain cells are made more susceptible to phagocytosis)
(3) C3 and C5 are the most important inflammatory mediators of the complement components
(a) Vascular phenomenon created by C3a, C5a, and to a lesser extent, C4a; stimulate histamine release from mast cells; called anaphylatoxins because they have similar effects in the reaction of anaphylaxis
(b) Leukocyte adhesion, chemotaxis, and activation occur through C5a as a chemotactic agent for neutrophils, monocytes, eosinophils, and basophils
(c) Phagocytosis enhanced by C3b, which acts as opsonin to facilitate foreign bodies being more efficiently engulfed by phagocytosis
(1) Generates vasoactive peptides from plasma proteins called kininogens by the action of proteases known as kallikreins
(2) Bradykinin increases vascular permeability; causes the contraction of smooth muscle, dilation of blood vessels, and pain
(3) Is triggered by the activation of Hageman factor (factor XII of the intrinsic clotting pathway)
(4) Is short lived; quickly inactivated by the enzyme kininase
(1) Induces the formation of thrombin, fibrinopeptides, and factor XII, which have inflammatory properties
(2) Activated by substances released during tissue destruction, such as collagen, proteinases, kallikrein, and bacterial endotoxins
(3) Prevents the spread of infection and inflammation; localizes microorganisms at the site of phagocytosis; helps clot formation to stop bleeding and for repair, chemotaxis of neutrophils, and increased permeability of vessels
(1) A lipid mediator that is a short-range hormone; is formed rapidly and acts locally
(2) Produces prostaglandins, leukotrienes, and lipoxins
(a) Prostaglandins, including PGE2, PGD2, PGF2a, PGI2, and TxA2, are most important in inflammation, causing increased permeability, the chemotactic effects of other mediators, and vasodilation resulting in edema as well as pain and fever in inflammation
(b) Pathway initiating these prostaglandins occurs by two different enzymes, COX-1 and COX-2
[1] COX-1 produces prostaglandins that are involved in inflammation and also maintains homeostasis
[2] COX-2 stimulates the production of prostaglandins involved in the inflammatory reactions noted above and may play a role in normal homeostasis
(c) Leukotrienes cause intense vasoconstriction, bronchospasm, and increased vascular permeability
(d) Lipoxins inhibit leukocyte recruitment, neutrophil chemotaxis, and adhesion to the endothelium and may play a role in resolving inflammation
(e) Resolvins inhibit leukocyte recruitment and activation by inhibiting the production of cytokines
(f) Platelet-activating factor (PAF) causes platelet stimulation, vasoconstriction, bronchoconstriction, vasodilation, and increased venular permeability; far more potent than histamine; increased leukocyte adhesion to endothelium; chemotaxis, degranulation, and oxidative burst; also boosts synthesis of other mediators
(g) Tumor necrosis factor and interleukin 1
[1] Two major cytokines that mediate inflammation and are produced primarily by macrophages
[2] Responsible for endothelial activation, which induces the synthesis of endothelial adhesion molecules and chemical mediators; producing enzymes associated with matrix remodeling; and increasing the surface thrombogenicity of endothelium
[3] Induces the systemic acute phase responses of infection and injury, including fever, loss of appetite, release of neutrophils, corticotropin, and corticosteroids
[4] TNF—responsible for the hemodynamic effects of septic shock, regulates body mass, and contributes to cachexia (wasting away) that is part of some infections and diseases
(h) Chemokines—small proteins that act as chemoattractants for leukocytes and control the normal migration of cells through various tissues
(i) Nitric oxide (NO)—released from endothelial cells; causes vasodilation by relaxing vascular smooth muscle; microbicidal; a mediator of host defense against infection
(j) Lysosomal components of leukocytes
[1] Neutrophils and monocytes, contain lysosomal granules; granules contain a variety of enzymes used for phagocytosis (such as lysozyme, collagenase, alkaline phosphatase, elastase)
[2] These enzymes are destructive, and if unchecked, can potentiate further inflammation and tissue damage
(k) Oxygen-derived free radicals may be released from leukocytes following a phagocytic event and are potent mediators
b. Neuropeptides, such as substance P and neurokinin A, play a role in the initiation and propagation of inflammatory response
Chronic Inflammation
1. Infiltration with macrophages, lymphocytes, and plasma cells
2. Tissue destruction, a hallmark of chronic inflammation
3. Attempts at healing by connective tissue replacement and fibrosis
1. Macrophages, lymphocytes, plasma cells, eosinophils, and mast cells
2. Produce granulomatous inflammation characterized by focal accumulation of activated macrophages that develops an epithelioid appearance
3. Products of these cells eliminate injurious agents and help initiate repair but are also responsible for much of the tissue injury in chronic inflammation
E Systemic effects of inflammation—acute phase response or the systemic inflammatory response syndrome
1. Fever—produced in response to pyrogens that act by stimulating prostaglandin synthesis
2. Acute phase proteins, including C-reactive protein (CRP), fibrinogen, and serum amyloid A protein (SAA) increase during the inflammatory process
a. Bind to microbial cell walls and act as opsonin and fix complement
b. Prolonged activation causes secondary amyloidosis in chronic inflammation
c. Elevated CRP used as a marker for increased risk of myocardial infarction in persons with coronary artery disease
a. Common feature in bacterial infections resulting in elevated to extremely high levels of different types of leukocytes, depending on the type of infection
b. Normal count of white blood cells is 4000 to 10,000/mm3 in blood
c. In an inflammatory reaction, particularly with an infection, the white blood cell count can increase to 15,000 or 20,000 and sometimes to extremely high levels of well over 40,000/mm3
a. In severe bacterial infections, large quantities of cytokines, particularly TNF and IL-1, cause thrombosis and coagulation
b. Eventually, hypoglycemia and cardiovascular failure occur, resulting in septic shock
c. Multiple organs can be affected by inflammation and intravascular thrombosis, resulting in organ failure
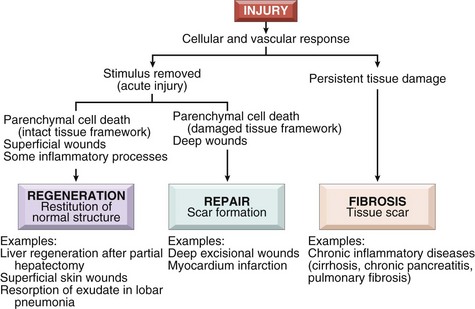
Regeneration and Wound Healing2
1. Regeneration—growth of cells and tissues to replace lost structures
2. Healing—a tissue response to a wound, inflammatory process, or cell necrosis in organs that cannot regenerate; involves either regeneration or scar formation (Figure 7-3)
1. Involves a complex process that includes a number of steps
a. An inflammatory response caused by the initial injury
b. Proliferation of parenchymal and connective tissue cells
c. Formation of new blood vessels (angiogenesis) and granulation tissue
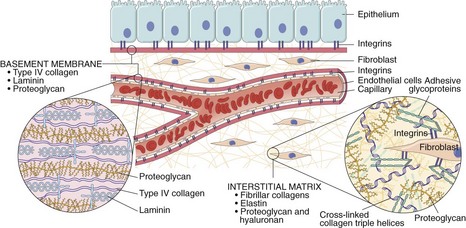
d. Synthesis of extracellular matrix (ECM) proteins and collagen deposition
2. Repair process—a combination of regeneration and scar formation that is influenced by local and systemic factors that may inhibit or prolong the wound healing process
3. Generally, repair begins early in inflammation—formation of granulation tissue, which is a hallmark of healing
1. Occurs from the branching and extension of adjacent blood vessels and the recruitment of endothelial progenitor cells (EPCs) from bone marrow
2. Angiogenesis from adjacent blood vessels occurs through the vasodilation and increased permeability of existing vessels, degradation of ECM, migration of endothelial cells, maturation of endothelial cells and remodeling into capillary tubes, and recruitment of periendothelial cells to support endothelial tubes and to form the mature vessel
3. Growth factors such as vascular endothelial growth factor (VEGF) support adult tissues undergoing angiogenesis
4. Directed migration of endothelial cells—controlled by ECM proteins, including integrins, matricellular proteins, and proteinases
D Scar formation—three processes occur (emigration and proliferation of fibroblasts in the site of injury, deposition of ECM, and tissue remodeling)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses