Connective Tissue Lesions
Fibrous Lesions
Reactive Hyperplasias
Although these lesions are all pathogenically related, different names or subdivisions have been devised because of variations in the anatomic site, clinical appearance, or microscopic picture. Those lesions that present as prominent red masses are discussed in Chapter 4.
Peripheral Fibroma
Clinical Features
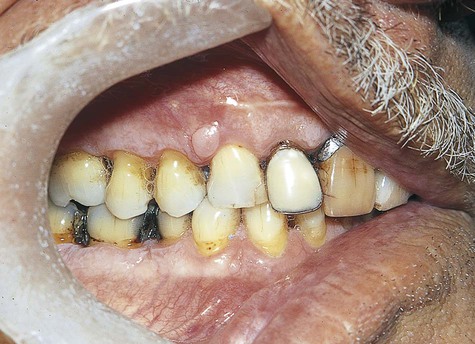
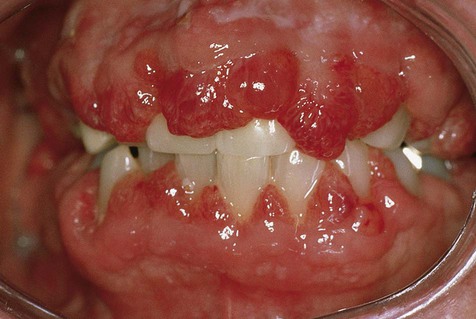
By definition, peripheral fibroma is a reactive hyperplastic mass that occurs on the gingiva and is believed to be derived from connective tissue of the submucosa or periodontal ligament (Figure 7-1). It may occur at any age, although it does have a predilection for young adults. Females develop these lesions more commonly than do males, and the gingiva anterior to the permanent molars is most often affected.
Histopathology
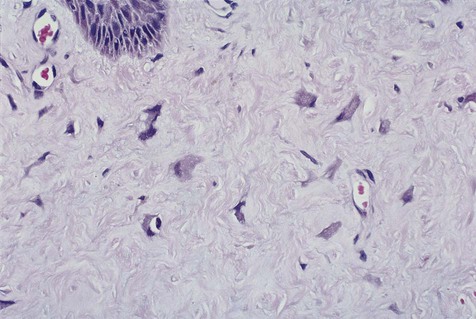
Peripheral ossifying fibroma is a gingival mass in which islands of woven (immature) bone and osteoid are seen. The bone is found within a lobular proliferation of plump, benign fibroblasts. Chronic inflammatory cells tend to be seen around the periphery of the lesion (Figure 7-2). The surface is typically ulcerated.
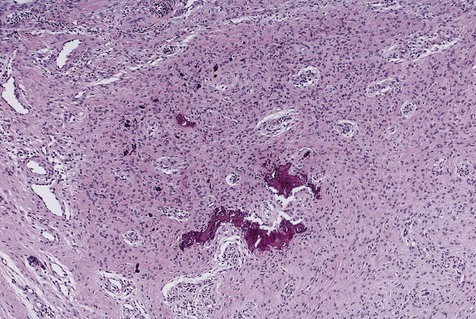
Note cellular fibroblastic proliferation with islands of new bone.
Giant cell fibroma is a fibrous hyperplasia in which many of the mesenchymal cells are relatively larger than normal fibroblasts (giant cells) and assume a stellate shape. Immunohistochemical studies have shown that most of these stellate cells are fibroblasts (a few factor XIIIa–positive dendritic cells are also typically present) (Figure 7-3). These same peculiar stellate cells can be found in focal fibrous hyperplastic lesions throughout the oral mucosa and occasionally on the skin (fibrous papule). One form of this lesion is known as retrocuspid papilla of the mandible.
Focal Fibrous Hyperplasia
Etiology
Focal fibrous hyperplasia is a reactive lesion usually caused by chronic trauma to oral mucous membranes. Overexuberant fibrous connective tissue repair results in a clinically evident submucosal mass. Although the terms traumatic fibroma and oral fibroma are often applied to these entities, they are misnomers because these lesions are not benign tumors of fibroblasts, as the term fibroma implies (Box 7-1).
Clinical Features
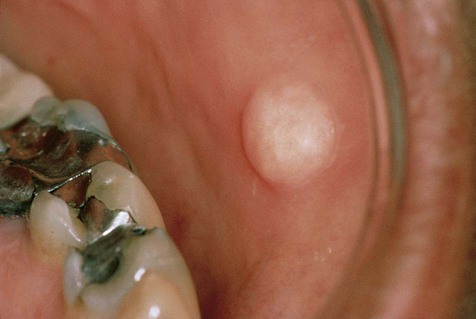
No gender or racial predilection for the development of this intraoral lesion has been noted. It is a very common reactive hyperplasia that is typically found in frequently traumatized areas, such as the buccal mucosa, the lateral border of the tongue, and the lower lip (Figure 7-4). It is a painless, broad-based swelling that is paler in color than the surrounding tissue because of its relative lack of vascular channels. The surface may occasionally be traumatically ulcerated, particularly in larger lesions. Lesions have limited growth potential and do not exceed 1 to 2 cm in diameter.
Histopathology
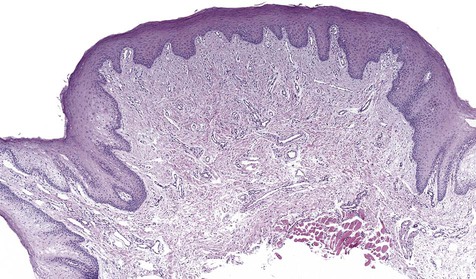
Collagen overproduction is the basic process that dominates the microscopy of this lesion. Fibroblasts are mature and widely scattered in a dense collagen matrix. Sparse chronic inflammatory cells may be seen, usually in a perivascular distribution (Figure 7-5). Overlying epithelium is often hyperkeratotic because of chronic low-grade friction.
Denture-Induced Fibrous Hyperplasia
Clinical Features
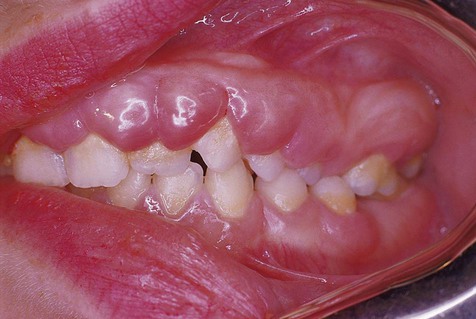
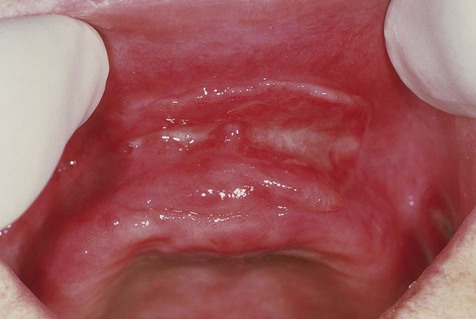
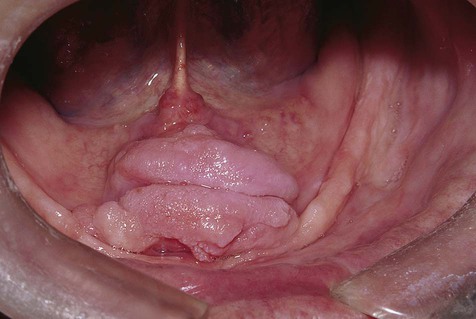
Denture-induced fibrous hyperplasia is a common lesion that occurs in the vestibular mucosa and less commonly along the mandibular lingual sulcus where the denture flange contacts tissue (Figures 7-6 and 7-7). As the bony ridges of the mandible and the maxilla resorb with long-term denture use, the flanges gradually extend farther into the vestibule. There, chronic irritation and trauma may incite an overexuberant fibrous connective tissue reparative response. The result is the appearance of painless folds of fibrous tissue surrounding the overextended denture flange.
Generalized Gingival Hyperplasia
Etiology
In generalized gingival hyperplasia, overgrowth of the gingiva may vary from mild enlargement of the interdental papillae to such severe uniform enlargement that the crowns of the teeth may be covered by hyperplastic tissue (Box 7-2). Uniform or generalized gingival fibrous connective tissue hyperplasia may be due to one of several etiologic factors. Most cases are nonspecific and are the result of an unusual hyperplastic tissue response to chronic inflammation associated with local factors such as plaque, calculus, or bacteria. Why only some patients have a propensity for the development of connective tissue hyperplasia in response to local factors is unknown. Recent studies have reported a possible role for keratinocyte growth factor (a member of the fibroblast growth factor family) in this condition.
Clinical Features
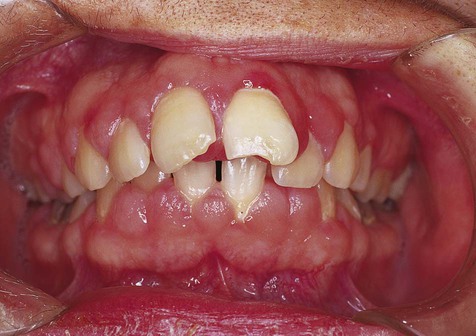
The clinical feature common to the variously caused gingival hyperplasias is an increase in bulk of the free and attached gingiva, especially the interdental papillae (Figures 7-8 to 7-10). Stippling is lost, and gingival margins become rolled and blunted. The consistency of the gingiva ranges from soft and spongy to firm and dense, depending directly on the density and amount of fibrosis. A range of color from red-blue to lighter than surrounding tissue is also seen; this varies with the severity of the inflammatory response as well. Generally, hyperplasias associated with nonspecific local factors and hormonal changes appear more inflamed clinically than drug-induced and idiopathic forms. The idiopathic type is particularly dense and fibrous, with relatively little inflammatory change.
Neoplasms
Solitary Fibrous Tumor
Solitary fibrous tumor is a benign proliferation of spindle cells of disputed but probable fibroblastic origin (Box 7-3). This lesion was first described as a tumor of the pleura and has subsequently been described at many other sites. Oral lesions are seen in adults and present as submucosal masses predominantly in the buccal mucosa (Box 7-4).
Microscopically, lesions are circumscribed and are composed of a “patternless” proliferation of spindle cells (Figure 7-11). Some areas may suggest neurofibroma or Schwannoma, whereas others may suggest hemangiopericytoma or leiomyoma. Tumor cells characteristically stain positive for CD34 (90% to 95% of cases), CD99 (70%), and Bcl-2 (20% to 35%) by immunohistochemistry. Many factor XIIIa–positive cells may be found. Immunohistochemistry has permitted a better understanding of this entity and more reliable identification; therefore many oral tumors previously diagnosed by light microscopy as other soft tissue neoplasms such as leiomyoma, hemangiopericytoma, and benign fibrous histiocytoma probably represent solitary fibrous tumor.
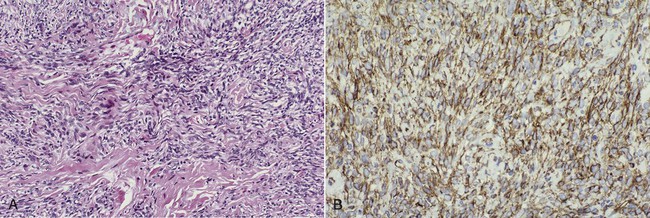
A, Haphazard spindle cell proliferation. B, Immunohistochemical stain for CD34 showing positive cytoplasmic staining (brown) of tumor cells.
Myxoma
Histopathology
Oral myxomas are not encapsulated and may exhibit infiltration into surrounding soft tissue. Dispersed stellate and spindle-shaped fibroblasts are found in a loose myxoid stroma. Soft tissue myxomas may be confused with other myxoid lesions, such as nerve sheath myxoma and oral focal mucinosis (Table 7-1).
TABLE 7-1
MUCOSAL MYXOID LESIONS: MICROSCOPIC DIFFERENTIATION
| Mast Cells | Reticulin | Pattern | Periphery | |
| Soft tissue myxoma | No | Yes | Diffuse, uniform | Blending, infiltration |
| Nerve sheath myxoma | Yes | Yes | Lobular | Condensed fibrous tissue |
| Focal mucinosis | No | No | Uniform | Circumscribed |

Nodular Fasciitis
Histopathology
A nodular growth contains plump spindle cells with vesicular nuclei in a haphazard to storiform arrangement (Figure 7-12). Myxoid areas are usually found. Multinucleated giant cells are occasionally present and may originate from adjacent muscle or from fusion of macrophages. Mitotic figures may be frequent but are morphologically normal in appearance. Inflammatory cells and extravasated red blood cells are also microscopic features of nodular fasciitis. By immunohistochemistry, the cells in nodular fasciitis express smooth m/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses