Periodontal Regeneration and Reconstructive Surgery
Richard T. Kao, Henry H. Takei, David L. Cochran and Marc L. Nevins
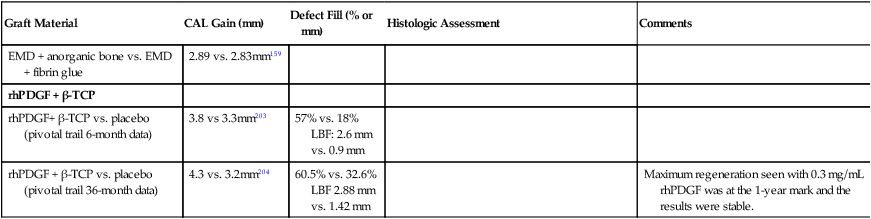
When the periodontium is damaged by inflammation or as a result of surgical treatment, the defect heals either through periodontal regeneration or repair.* In periodontal regeneration, healing occurs through the reconstitution of a new periodontium, which involves the formation of alveolar bone, functionally aligned periodontal ligament, and new cementum. Alternatively, repair due to healing by replacement with epithelial and/or connective tissue that matures into various nonfunctional types of scar tissue, is termed new attachment. Histologically, patterns of repair include long junctional epithelium, ankylosis, and/or new attachment (see Chapter 1). Although the stability of periodontal repair is not clear, the ideal goal of periodontal surgical therapy is periodontal regeneration.
Assessment of Periodontal Wound Healing
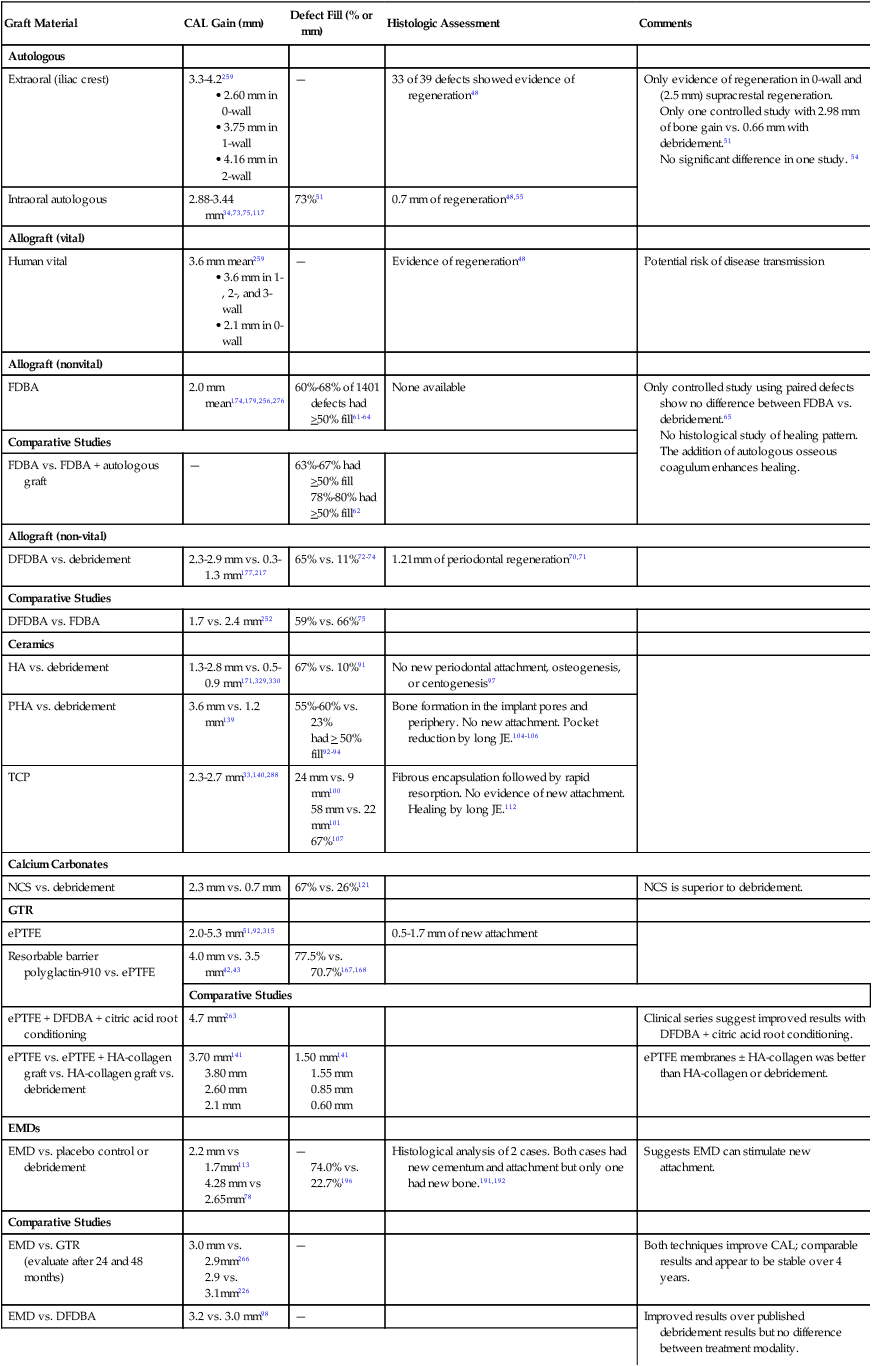
It is sometimes difficult in clinical and experimental situations to determine whether regeneration or new attachment has occurred and the extent to which it has occurred. Although there are various evidences of reconstruction, the proof of principle for the type of healing is determined by histological studies. Once defined, the evidence found subsequently by clinical, radiographic, and surgical reentry findings is implied.35,37,166 All these methods have advantages and shortcomings that should be well understood and considered in individual cases and when critically evaluating the literature. A comparative analysis of regenerative approaches is detailed in Table 61-1.
TABLE 61-1
Comparative Analysis of Regenerative Approaches
| Graft Material | CAL Gain (mm) | Defect Fill (% or mm) | Histologic Assessment | Comments |
| Autologous | ||||
| Extraoral (iliac crest) | 3.3-4.2259 |
Only one controlled study with 2.98 mm of bone gain vs. 0.66 mm with debridement.51
No significant difference in one study. 54
No histological study of healing pattern.
The addition of autologous osseous coagulum enhances healing.
78%-80% had >50% fill62
had > 50% fill92–94
58 mm vs. 22 mm101
67%107
polyglactin-910 vs. ePTFE
3.80 mm
2.60 mm
2.1 mm
1.55 mm
0.85 mm
0.60 mm
4.28 mm vs 2.65mm78
74.0% vs. 22.7%196
(evaluate after 24 and 48 months)
2.9 vs. 3.1mm226
LBF: 2.6 mm vs. 0.9 mm
LBF 2.88 mm vs. 1.42 mm
Histological Methods
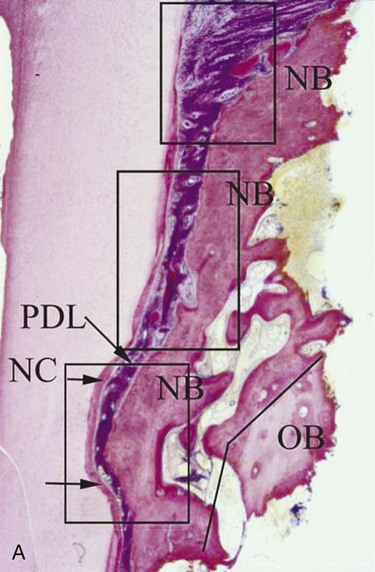
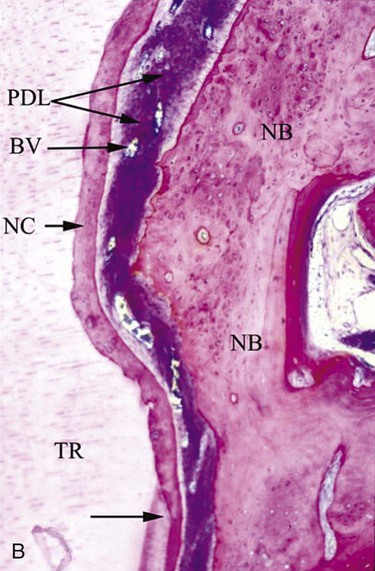
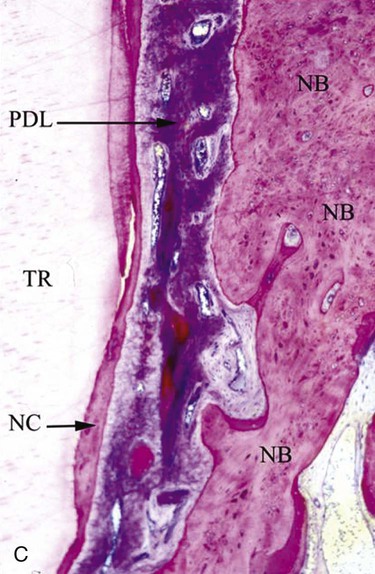
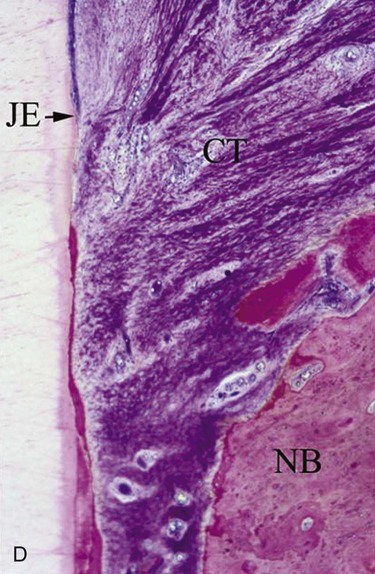
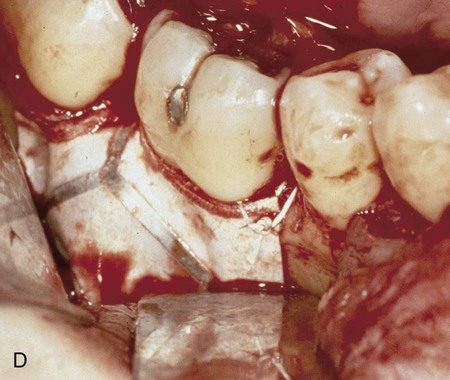
It is only through histological analysis can one define the nature of the reparative tissue (Figure 61-1). In periodontal reconstructive surgery, the goal is to achieve periodontal regeneration. Classically, experimental animal model systems36,213 are used whereby reference notches are placed at the base of bony defects or at the apical extent of calculus deposits. Periodontal regeneration is considered to have occurred when the newly formed functionally aligned periodontium is coronal to the apical extent of the notches. Reparative tissue response dominated may include long junctional epithelium, connective tissue adhesion, and root resorption associated with ankylosis. It should be noted that the healing may be predominated by periodontal regeneration; there may be localized areas of repair.233 Unfortunately, this approach cannot be used in human studies as it would be unethical to extract the treated tooth, especially when it responded positively to therapy. On rare circumstances, human histology is available if the tooth is to be extracted in conjunction with orthodontic or restorative therapy (Figure 61-2).
Clinical Methods
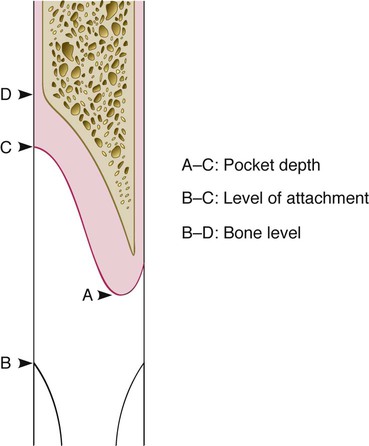
Clinical methods to evaluate periodontal reconstruction consist of comparisons between pretreatment and posttreatment pocket probings and determinations of clinical gingival findings. The probe can be used to determine pocket depth, attachment level, and bone level (Figure 61-3). Clinical determinations of attachment level are more useful than probing pocket depths because the latter may change as a result of displacement of the gingival margin (see Chapter 29).
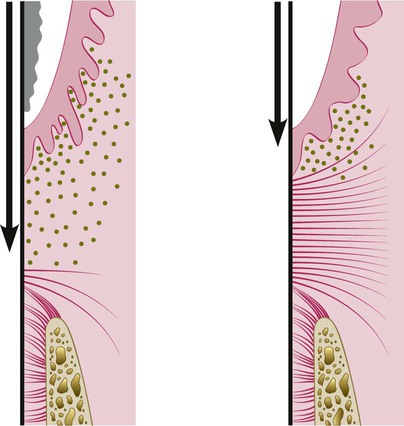
Several studies have determined that the depth of penetration of a probe in a periodontal pocket varies according to the degree of inflammatory involvement of the tissues immediately beneath the bottom of the pocket (Figure 61-4). Therefore, even though the forces used may be standardized with pressure-sensitive probes, there is an inherent margin of error in this method that is difficult to overcome. Fowler et al71 have calculated this error to be 1.2 mm, but it is even greater when furcations are probed.189
Bone probing performed with the patient under anesthesia is not subject to this error and has been found to be as accurate as bone height measurements made on surgical reentry.93,240,311
Measurements of the defect should be made before and after treatment from the same point within the defect and with the same angulation of the probe. This reproducibility of probe placement is difficult and may be facilitated in part by using a grooved stent to guide the introduction of the probe (Figure 61-5). Preoperative and postoperative comparability of probing measurements that do not use this standardized method may be open to question.
Radiographic Methods
Radiographic evaluation of periodontal regeneration allows assessment of the bone tissue adjacent to the tooth. This technique also requires carefully standardized techniques for reproducible positioning of the film and the tube.216,249 Even with standardized techniques (see Chapter 31), the radiograph does not show the entire topography of the area before or after treatment. Furthermore, thin bone trabeculae may exist before treatment and go undetected radiographically because a certain minimal amount of mineralized tissue must be present to register on the radiograph. Several studies have demonstrated that radiographs, even those taken with standardized methods, are less reliable than clinical probing techniques.154,299 A comparative study of pretreatment bone levels and posttherapy bone fill with 12-month reentry bone measurements showed that linear radiographic analysis significantly underestimates pretreatment bone loss and posttreatment bone fill.300
Studies with subtraction radiography have enhanced the usefulness of radiographic evaluation.60,61,317 A comparative study of linear measurement, computer-assisted densitometric image analysis (CADIA), and a method combining the two reported that the linear-CADIA method offers the highest level of accuracy.303
Surgical Reentry
The surgical reentry of a treated defect after a period of healing can provide a good view of the state of the bone crest that can be compared with the view taken during the initial surgical intervention and can also be subject to measurements (Figure 61-6). Models from impressions of the bone taken at the initial surgery and later at reentry can be used to assess the results of therapy.
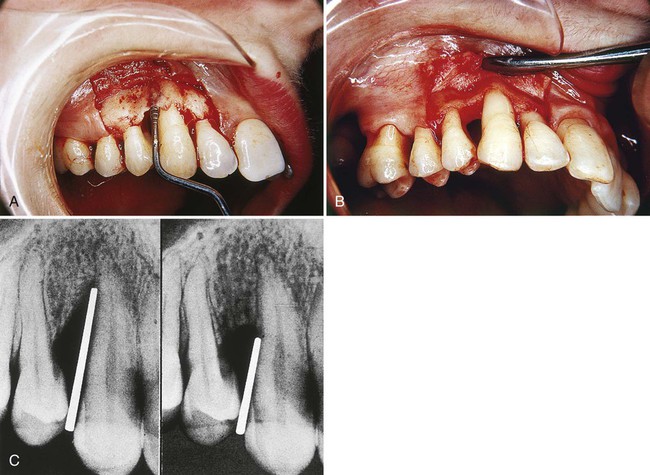
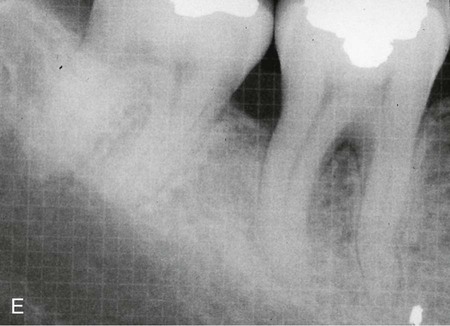
This method is very useful but has two shortcomings: It requires a frequently unnecessary second procedure, and it does not show the type of attachment that exists (i.e., new attachment or long junctional epithelium35) (Figure 61-7).
Reconstructive Surgical Techniques
Reconstructive techniques can be subdivided into three major therapeutic approaches: non–bone graft–associated, graft-associated, and biological mediator–associated new attachment and regeneration. In clinical practice, it is common for clinicians to combine these various approaches.84,104
All recommended techniques include careful case selection and complete removal of all irritants on the root surface. Although this can be done in some cases as a closed procedure, in the great majority of cases, it should be done after exposure of the area with a flap.6,7 Flap design and incisions should follow the description given in Chapter 59 for flaps used in reconstructive surgery. Trauma from occlusion, as well as other factors, may impair posttreatment healing of the supporting periodontal tissues, reducing the likelihood of new attachment. Occlusal adjustment, if needed, is therefore indicated.
Systemic antibiotics are generally used after reconstructive periodontal therapy, although definitive information on the advisability of this measure is still lacking. Case reports have shown extensive reconstruction of periodontal lesions after scaling, root planing, and curettage, with systemic and local treatment using penicillin or tetracycline, in combination with other forms of therapy.32,192
Non–Graft-Associated Reconstructive Procedures
Guided Tissue Regeneration.
GTR is used for the prevention of epithelial migration along the cemental wall of the pocket and maintaining space for clot stabilization. Derived from the classic studies of Nyman, Lindhe, Karring, and Gottlow, this method is based on the assumption that periodontal ligament and perivascular cells have the potential for regeneration of the attachment apparatus of the tooth.* GTR consists of placing barriers of different types (membranes) to cover the bone and periodontal ligament, thus temporarily separating them from the gingival epithelium and connective tissue. Excluding the epithelium and the gingival connective tissue from the root surface during the postsurgical healing phase not only prevents epithelial migration into the wound but also favors repopulation of the area by cells from the periodontal ligament and the bone38 (see also Chapter 1). It should be noted that in the United States, GTR is often performed with some type of bone graft as a scaffolding agent, so it is a combined therapy. In Europe and other parts of the world where regulatory and religious constraints makes human graft materials unavailable, so it is performed as a traditional GTR procedure and may be occasionally used in conjunction with a other graft materials as a combined therapy.
Initial animal experiments using Millipore filters and Teflon membranes resulted in regeneration of cementum and alveolar bone and a functional periodontal ligament.32,34,38,129 Clinical case reports indicate that GTR results in a gain in attachment level.17,18 Histologic studies in humans provided evidence of periodontal reconstruction in most cases, even with horizontal bone loss.90,290,292
The use of polytetrafluoroethylene (PTFE) membranes has been tested in controlled clinical studies in mandibular molar furcations and has shown statistically significant decreases in pocket depths and improvement in attachment levels after 6 months, but bone level measurements have been inconclusive.158,225 A study on maxillary molar furcations did not result in significant gain in attachment or bone levels.182
With the regenerative success associated with the use of nonresorbable membrane, the advantage and disadvantage of this approach became apparent. Notably, problems such as membrane exposure, which resulted in no or limited regeneration and the need for a secondary procedure for surgical removal resulted in the development of biodegradable membranes.274 Today in clinical practice, most GTR procedures use biodegradable membranes while the nonresorbable membranes are used for implant site development. Nevertheless, the historical research using nonresorbable membranes and the development of various types of biodegradable membranes is valuable.
Nonresorbable Membranes.
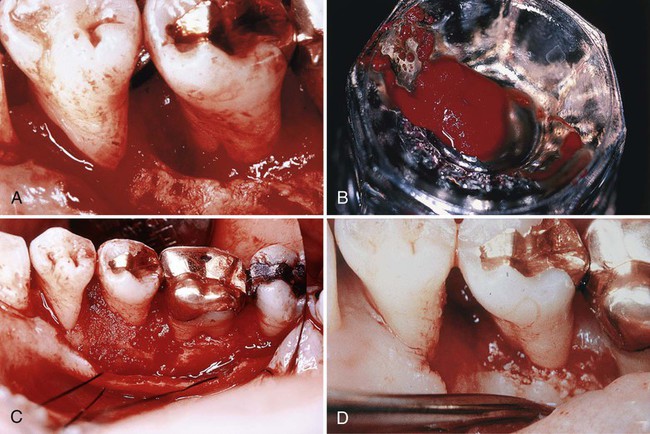
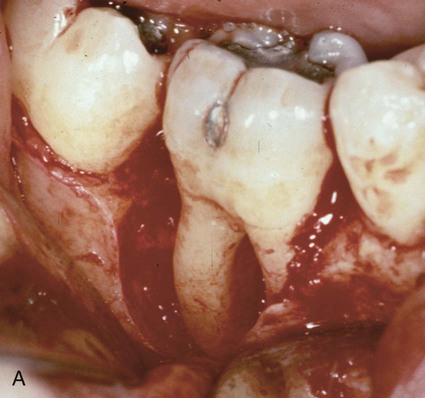
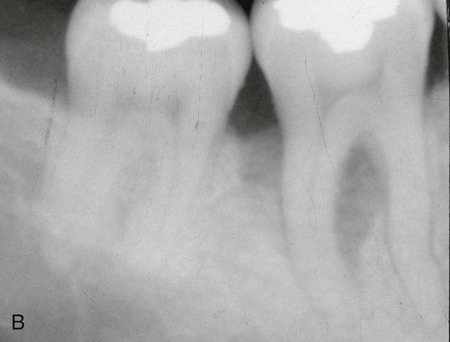
The clinical effectiveness of ePTFE membranes is dependent on technique. Preservation of the keratinized gingiva and a relatively thick overlying surgical flap are critical to avoid perforation of the flap by the membrane during healing. After the surgical area has been flapped, the defect is degranulated and the root surface scaled and root planed. The ePTFE membrane is trimmed to adapt to tooth configuration, secured by ePTFE sutures, and the flap repositioned. It is interesting to note that, although much of the emphasis in the literature is on adapting the membrane to the defect, no membrane can ever be perfectly adapted. Despite gaps, these membranes seem to work. After membrane placement, healing is allowed to proceed for 4 to 6 weeks. Barring any membrane exposure, a second surgery is performed to remove the membrane. During this removal, the healing tissue appears reddish and granulomatous. After membrane removal, the area should not be probed for 3 months. Radiographic evidence of bone fill is usually present after 6 months and should continue over the course of 1 year (Figure 61-8).
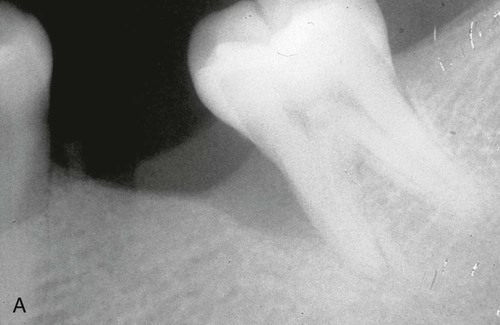
Clinical studies have shown that ePTFE membranes used in GTR procedures are more effective than surgical debridement in correcting intrabony defects.* In intrabony and furcation defects, there are gains in clinical attachment level (3 to 6 mm), improved bone levels (2.4 to 4.8 mm), and probing depth reductions (3.5 to 6 mm). Studies have demonstrated that these regenerative results can be maintained over the course of several years.51,92,263,315
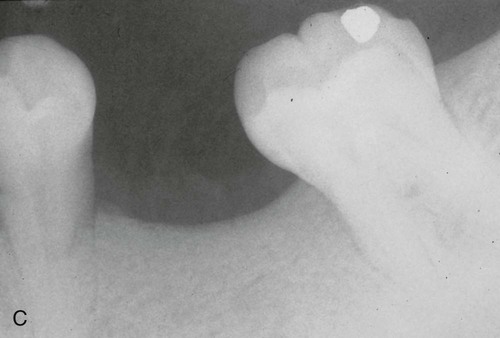
The advent of titanium-reinforced ePTFE allowed for the formation of larger spaces, thus permitting correction of larger defects (Figure 61-9).50 This resulted in significant clinical improvements using titanium-reinforced ePTFE compared to ePTFE.
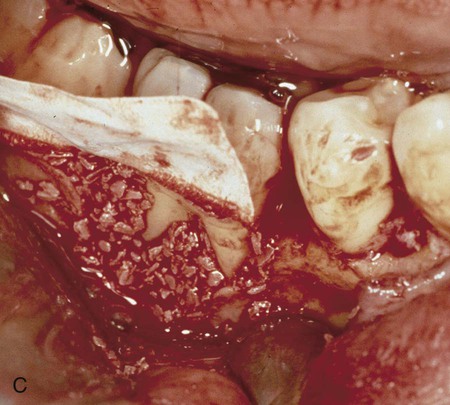
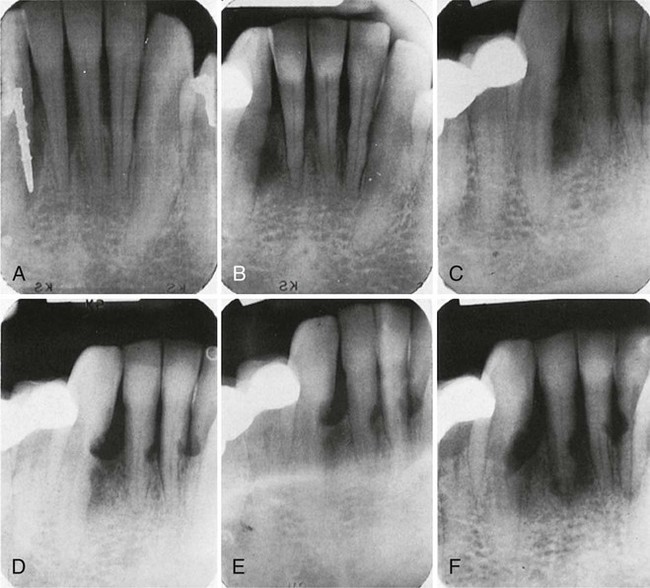
To determine how regeneration can be enhanced with GTR technique, the prolonged retention of ePTFE membranes was evaluated.196 After allowing the membrane to be retained for 4 months, surgical re-entry after 1 year determined that the mean bone fill of intrabony defects was 95%. This suggests that prolonged retention of a barrier membrane is desirable if no tissue perforation is present. This is consistent with many clinical reports of the improved bone quality associated with guided bone regeneration in implant site development.
The major problem with using non-resorbable membranes is that the membrane is exposed to the oral environment during healing. Upon exposure, the membrane is contaminated and colonized by oral microflora.187,206,213,281 Several studies have shown that contamination of the surgical field can result in decreased formation of new attachment. If the membrane is exposed, the infection can be temporarily managed with topical application of chlorhexidine. This may minimize the infection and extend the time the membrane can be retained in place.
Biodegradable Membranes.
A recent GTR study compared the use of bioresorbable membranes (polylactate/polygalactate copolymer) versus ePTFE membranes, with surgical debridement as a control.52 After 1 year, significant gains in clinical attachment level (CAL) were observed in all three groups. There was no difference in CAL gain between the two membrane groups, with both of them gaining 2 mm or more. In both membrane groups, 83% of the sites improved 4 mm or more, which was significantly better than the surgical debridement control group. These findings indicate GTR procedures are equally effective using resorbable and nonresorbable membranes. This finding has been confirmed by other investigators.42,43,316
A large multicenter clinical study reported the use of bioresorbable membranes in 203 consecutively treated intrabony defects.66 After 1 year, investigators found that CAL improved by 79%, and 78% of the sites improved by 4 mm or more. An average of 3 mm of bone fill was measured radiographically. Compromised clinical results occurred in incidences in which membranes were exposed or patients had poor plaque control.
The search for resorbable membranes has included trials and tests with numerous materials and collagens from different species such as bovine, porcine, Cargile membrane derived from the cecum of an ox, polylactic acid, Vicryl (polyglactin 910), synthetic skin (Biobrane), and freeze-dried dura mater.* Clinical studies with a mixture of copolymers derived from polylactic acid and acetyl tributyl citrate resorbable membranes (Guidor membrane, no longer on the market) and a poly-d,l-lactide-co-glycolide (Resolut membrane, also no longer on the market) have shown significant gains in clinical attachment and bone fill.43,68,198
The potential of using autogenous periosteum as a membrane and also to stimulate periodontal regeneration has been explored in two controlled clinical studies, one of grade II furcation involvements in mandibular molars and another of interdental defects.153,157 The periosteum was obtained from the patient’s palate by means of a window flap. Both studies reported that autogenous periosteal grafts can be used in GTR and result in significant gains in clinical attachment and osseous defect fill.
Laser-Assisted New Attachment Procedure.
The role of laser in periodontal therapy remains controversial (see Chapter 65). Nevertheless, the use of neodymium : yttrium-aluminum-garnet (Nd : YAG) to perform surgical LANAPs has been reported for the management of chronic periodontitis138,186 and can potentially result in new attachment and periodontal regeneration (see Chapter 65).201,334
Non–Graft-Associated Procedures of Historical Interest
Removal of Junctional and Pocket Epithelium.
Since the earliest attempts at periodontal new attachment, the presence of junctional and pocket epithelium has been perceived as a barrier to successful therapy because its presence interferes with the direct apposition of connective tissue and cementum, thus limiting the height to which periodontal fibers can insert to the cementum.86,131,156,212 Several methods have been recommended to remove the junctional and pocket epithelium. These include curettage, chemical agents, ultrasonics, lasers, and surgical techniques.
Curettage.
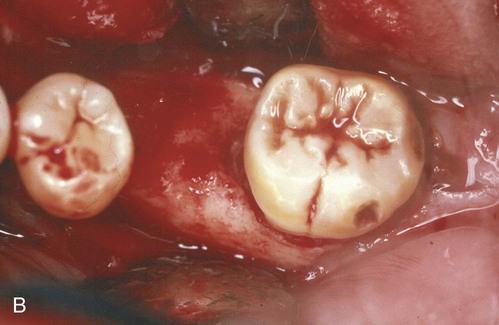
Results of removal of the epithelium by means of curettage vary from complete removal to persistence of as much as 50%.283 Though curettage is not a reliable procedure, occasional bone regeneration does occur (Figure 61-10). Ultrasonic methods, lasers, and rotary abrasive stones have also been used, but their effects cannot be controlled because of the clinician’s lack of vision and tactile sense when using these methods.
Biomodification of Root Surface.
Citric Acid.
The following actions of citric acid have been reported:
1. Accelerated healing and new cementum formation occur after surgical detachment of the gingival tissues and demineralization of the root surface by means of citric acid.238
2. Topically applied citric acid on periodontally diseased root surfaces has no effect on nonplaned roots, but after root planing, the acid produces a 4-µm-deep demineralized zone with exposed collagen fibers.82
3. Root-planed, non–citric acid treated roots are left with a surface smear layer of microcrystalline debris. Citric acid application not only removes the smear layer, exposing the dentinal tubules, but also makes the tubules appear wider and with funnel-shaped orifices.224
4. Citric acid has also been shown in vitro to eliminate endotoxins and bacteria from the diseased tooth surface.55,70
5. An early fibrin linkage to collagen fibers exposed by the citric acid treatment prevents the epithelium from migrating over treated roots.223
This technique using citric acid has been extensively investigated in animals and humans. Studies in dogs have shown encouraging results, especially for the treatment of furcation lesions, but the results in humans have been contradictory.*
The recommended citric acid technique is as follows:
1. Raise a mucoperiosteal flap and thoroughly instrument the root surface, removing calculus and underlying cementum.
2. Apply cotton pledgets soaked in a saturated solution of citric acid (pH of 1.0) for 2 to 5 minutes.
3. Remove pledgets, and irrigate root surface profusely with water.
The use of citric acid has also been recommended in conjunction with coverage of denuded roots using free gingival grafts (see Chapter 63).
Fibronectin.
Fibronectin is the glycoprotein that fibroblasts require to attach to root surfaces. The addition of fibronectin to the root surface may promote new attachment.28,69,297 However, increasing fibronectin above plasma levels produces no obvious advantages. Adding fibronectin and citric acid to lesions treated with GTR in dogs did not improve the results.29,284
Tetracycline.
In vitro treatment of the dentin surfaces with tetracycline increases binding of fibronectin, which in turn stimulates fibroblast attachment and growth while suppressing epithelial cell attachment and migration.287,298 Tetracycline also removes an amorphous surface layer and exposes the dentin tubules. In vivo studies, however, have not shown favorable results.318,319 A human study showed a trend for greater connective tissue attachment after tetracycline treatment of roots. Tetracycline gave better results when used alone than when combined with fibronectin.3 Tetracycline has been used as an adjunctive procedure in preparation of the root in regenerative procedures and is recommended step for use with biologic mediators.
Surgical Techniques.
Surgical techniques have been recommended to eliminate the pocket and junctional epithelium. The excisional new attachment procedure (ENAP) consists of an internal bevel incision performed with a surgical knife, followed by removal of the excised tissue.328 No attempt is made to elevate a flap. After careful scaling and root planing, interproximal sutures are used to close the wound (see Chapter 56). This approach has been modified and is used in conjunction with the Nd : YAG laser in the previously described LANAP procedure.
Glickman88 and Prichard228 have advocated performing a gingivectomy to the crest of the alveolar bone and debriding the defect. Excellent results have been obtained with this technique in uncontrolled human studies.16,230
The modified Widman flap, as described by Ramfjord and Nissle,234 is similar to the excisional new attachment procedure but is followed by elevation of a flap for better exposure of the area. The internal bevel incision eliminates the pocket epithelium (see Chapter 59).
Preventing or Impeding the Epithelial Migration.
For experimental purposes, several investigators have analyzed in animals and humans the effect of excluding the epithelium by amputating the crown of the tooth and covering the root with the flap (“root submergence”).19,20,22,39 This experimental technique not only excludes the epithelium but also prevents microbial contamination of the wound during the reparative stages. Successful repair of osseous lesions in the submerged environment was reported, but obviously this method has little or no clinical application.
Two other methods have been proposed to prevent or impede the migration of the epithelium. One consists of the total removal of the interdental papilla covering the defect and its replacement with a free autogenous graft obtained from the palate.63 During healing, the graft epithelium necroses and is slowly replaced by proliferating epithelium from the gingival surface. The graft simply delays the epithelium from proliferating into the healing area. This method has not been widely used.
The second approach is the use of coronally displaced flaps, which increase the distance between the epithelial wound edge and the healing area. This technique is particularly suitable for the treatment of mandibular molar furcations and has been used mostly in conjunction with citric acid treatment of the roots.81,167 Periodontal regeneration after the use of this technique has been demonstrated histologically in humans.276
Clot Stabilization, Wound Protection, and Space Creation.
Some investigators have attributed the successful results reported with graft materials, barrier membranes, and coronally displaced flaps to the fact that these techniques protect the wound and create a space for undisturbed and stable maturation of the clot83,156,157 This hypothesis suggests that preservation of the root surface fibrin clot interface prevents apical migration of the gingival epithelium and allows for connective tissue attachment during the early wound-healing period.83,213,223
The importance of space creation for bone repair has long been recognized in orthopedic and maxillofacial surgery. Transference of this concept to periodontal therapy has been explored for treatment of periodontal and periimplant osseous defects and for root coverage. The space can be created by using a titanium-reinforced ePTFE membrane to prevent its collapse. For the study of reconstructive techniques, these membranes were placed over experimentally created supraalveolar bone defects in dogs, and considerable bone reconstruction was reported.278 The following is a discussion of the guided tissue regeneration technique.
Graft Materials and Procedures
Numerous therapeutic grafting modalities for restoring periodontal osseous defects have been investigated. Periodontal reconstruction can be attained without the use of bone grafts in meticulously treated three-wall defects (intrabony defects) and in periodontal and endodontic abscesses.32,116,137,230 New attachment is more likely to occur when the destructive process has occurred rapidly, such as after treatment of pockets complicated by acute periodontal abscesses and after treatment of acute necrotizing ulcerative lesions.197 The use of graft materials at one time was to provide regenerative inductive effect but should be viewed primarily as providing a scaffold for healing.
Periodontal defects as sites for transplantation differ from osseous cavities surrounded by bony walls. Saliva and bacteria may easily penetrate along the root surface, and epithelial cells may proliferate into the defect, resulting in contamination and possible exfoliation of the grafts. Therefore the principles established to govern transplantation of bone or other materials into closed osseous cavities are not fully applicable to transplantation of bone into periodontal defects.62
Schallhorn262 defined the considerations that govern the selection of a material as follows: biologic acceptability, predictability, clinical feasibility, minimal operative hazards, minimal postoperative sequelae, and patient acceptance. It is difficult to find a material with all these characteristics, and to date, there is no ideal material or technique.
All grafting techniques require presurgical scaling, occlusal adjustment as needed, and exposure of the defect with a full-thickness flap. The flap technique best suited for grafting purposes is the papilla preservation flap because it provides complete coverage of the interdental area after suturing (see Chapter 57). The use of antibiotics after the procedure is generally recommended.
Autogenous Bone Grafts
Bone from Intraoral Sites.
In 1923, Hegedüs112 attempted to use bone grafts for the reconstruction of bone defects produced by periodontal disease. The method was revived by Nabers and O’Leary198 in 1965, and numerous efforts have been made since that time to define its indications and technique.
Sources of bone include bone from healing extraction wounds, bone from edentulous ridges, bone trephined from within the jaw without damaging the roots, newly formed bone in wounds especially created for the purpose, bone removed from tuberosity, and the ramus and bone removed during osteoplasty and ostectomy.34,103,116,117,246,327
Osseous Coagulum.
Robinson described a technique using a mixture of bone dust and blood that he termed “osseous coagulum.”246 The technique uses small particles ground from cortical bone. The advantage of the particle size is that it provides additional surface area for the interaction of cellular and vascular elements.
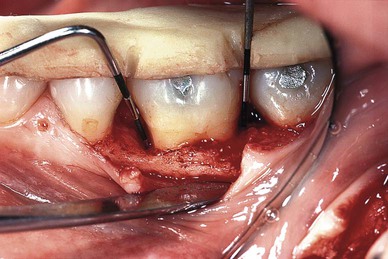
Sources of the graft material include the lingual ridge on the mandible, exostoses, edentulous ridges, the bone distal to a terminal tooth, bone removed by osteoplasty or ostectomy, and the lingual surface of the mandible or maxilla at least 5 mm from the roots. Bone is removed with a carbide bur #6 or #8 at speeds between 5000 and 30,000 rpm, placed in a sterile dappen dish and used to fill the defect (Figure 61-11). The obvious advantage of this technique is the ease of obtaining bone from an area already exposed during surgery. The disadvantages are its relatively low predictability and the inability to procure adequate material for large defects.63 Although notable success has been reported by many individuals, studies documenting the efficacy of the technique are still inconclusive.53,74,79,245
Bone Blend.
Some disadvantages of osseous coagulum derive from the inability to use aspiration during accumulation of the coagulum. Another problem is the unknown quantity and quality of the bone fragments in the collected material. To overcome these problems, the “bone blend technique” has been proposed.56
The bone blend technique uses an autoclaved plastic capsule and pestle. Bone is removed from a predetermined site, triturated in the capsule to a workable, plasticlike mass, and packed into bony defects. Froum et al73–75 found osseous coagulum–bone blend procedures to be at least as effective as iliac autografts and open curettage.
Cancellous Bone Marrow Transplants.
Cancellous bone can be obtained from the maxillary tuberosity, edentulous areas, and healing sockets.110 The maxillary tuberosity frequently contains abundant cancellous bone, particularly if the third molars are not present. After a ridge incision is made distally from the last molar, bone is removed with a curved rongeur. Care should be taken not to extend the incision too far distally to avoid entering the mucosal tissue of the pharyngeal area. Also, the location of the maxillary sinus must be analyzed on the radiograph to avoid entering or disturbing it.
Bone from Extraoral Sites.
The use of fresh or preserved iliac cancellous marrow bone has been extensively investigated. This material has been used by orthopedic surgeons for years. Data from human and animal studies support its use, and the technique has proved successful in osseous defects with various numbers of walls. It has also been successful in furcations and even supracrestally to some extent.10,26,58,259,260 However, because of numerous problems associated with its use, the technique is no longer in use.260 Some of the problems were postoperative infection, bone exfoliation, sequestration, varying rates of healing, root resorption, and rapid recurrence of the defect (Figure 61-12). Other problems were increased patient expense and difficulty in procuring the donor material.22,50,174,175
Bone Allografts.
Obtaining donor material for autograft purposes necessitates inflicting surgical trauma on another part of the patient’s body. Obviously, it would be to the patient’s and therapist’s advantage if a suitable substitute could be used for grafting purposes that would offer similar potential for repair and not require the additional surgical removal of donor material from the patient. However, both allografts and xenografts are foreign to the patient and, therefore, have the potential to provoke an immune response. Attempts have been made to suppress the antigenic potential of allografts and xenografts by radiation, freezing, and chemical treatment.25
Numerous steps are also taken to eliminate viral infectivity. These include exclusion of donors from known high-risk groups and various tests on the cadaver tissues to exclude individuals with any type of infection or malignant disease. The material is then treated with chemical agents or strong acids to inactivate the virus, if still present. The risk of human immunodeficiency virus (HIV) infection has been calculated as 1 in 1 to 8 million and is therefore characterized as highly remote.178
Freeze-Dried Bone Allograft.
Several clinical studies by Mellonig, Bowers, and coworkers reported bone fill exceeding 50% in 67% of the defects grafted with freeze-dried bone allograft (FDBA) and in 78% of the defects grafted with FDBA plus autogenous bone.174,198,256,276 FDBA, however, is considered an osteoconductive material, whereas demineralized FDBA (DFDBA) is considered an osteoinductive graft. Laboratory studies have found that DFDBA has a higher osteogenic potential than FDBA and is therefore preferred.175,176,179
Demineralized Freeze-Dried Bone Allograft.
Experiments by Urist307–310 have established the osteogenic potential of DFDBA. Demineralization in cold, diluted hydrochloric acid exposes the components of bone matrix, which are closely associated with collagen fibrils and have been termed bone morphogenetic proteins (BMPs).41,309
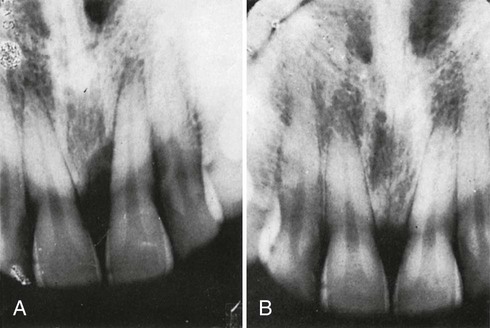
In 1975, Libin et al161 reported three patients with 4 to 10 mm of bone regeneration in periodontal osseous defects. Subsequent clinical studies were made with cancellous DFDBA and cortical DFDBA.217,231 The latter resulted in more desirable results (2.4 mm versus 1.38 mm of bone fill).
Bowers et al,22 in a histologic study in humans, showed new attachment and periodontal regeneration in defects grafted with DFDBA. Mellonig et al175,176,177 tested DFDBA against autogenous materials in the calvaria of guinea pigs and showed it to have similar osteogenic potential.
These studies provided strong evidence that DFDBA in periodontal defects results in significant probing depth reduction, attachment level gain, and osseous regeneration. The combination of DFDBA and GTR has also proved to be very successful9,263; however, limitations of the use of DFDBA include the possible, although remote, potential of disease transfer from the cadaver.
A bone-inductive protein isolated from the extracellular matrix of human bones, termed osteogenin or BMP-3, has been tested in human periodontal defects and seems to enhance osseous regeneration.23 This bone inductive protein is discussed later in this chapter.
Xenografts.
Calf bone (Boplant), treated by detergent extraction, sterilized, and freeze-dried, has been used for the treatment of osseous defects.10,258 Kiel bone is calf or ox bone denatured with 20% hydrogen peroxide, dried with acetone, and sterilized with ethylene oxide. Anorganic bone is ox bone from which the organic material has been extracted by means of ethylenediamine; it is then sterilized by autoclaving.173 These materials have been tried and discarded for various reasons.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses