Manufacturing and Distribution of N2O and O2 Gases
CHAPTER OBJECTIVES
Upon completion of this chapter, the reader should be able to:
1. Understand the simplicity of the nitrous oxide manufacturing process.
2. Recognize the primary users of nitrous oxide.
3. Recognize the regulatory agencies responsible for the oversight of nitrous oxide.
4. Identify how nitrous oxide is stored.
5. Identify properties of nitrous oxide and oxygen cylinders.
1 Nitrous Oxide Manufacturing
A. Process and Storage
1. The manufacturing process for N2O is relatively simple. The raw ingredient, ammonium nitrate (NH4NO3), is supplied as a clear liquid (liquid ammonium nitrate, LAN) or as solid, pellet-sized particles (solid ammonium nitrate, SAN). This material is used for fertilizer and as a primary ingredient of explosives.1
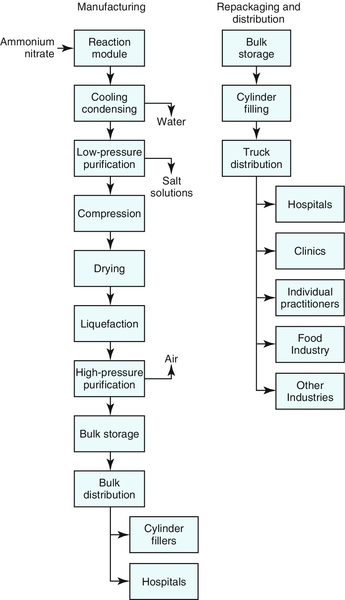
2. To make N2O, NH4NO3 is heated to approximately 250° C. At that point the NH4NO3 decomposes into N2O, water (steam), and some contaminants (NH4NO3 →N2O + 2H2O). The gas mixture is cooled to ambient room temperature, the steam is condensed, and most of the water is removed. The resulting crude N2O gas mixture is scrubbed to extract the contaminants. The gas is then compressed, dried to remove the remaining water, cooled, and liquefied. The resulting product is nearly pure (99.5% to 99.9%) and is stored as a liquefied, compressed gas at approximately 300 psi and 0° to 10° F in insulated and refrigerated storage tanks.1 Figure 6-1 is a flowchart of the steps involved in manufacturing, repackaging, and distributing N2O.
3. The manufactured product is kept refrigerated until it is directly transferred by insulated tanker trucks to hospitals with their own large storage facilities or to other gas repackagers and distributors. The overall medical marketing and distribution system is very effectively and efficiently deployed in the industry.
B. Users of N2O
1. Approximately 90% of the N2O produced by the major manufacturing companies is used in health settings. Most of this amount (80% to 85%) is used by hospitals to facilitate general anesthesia. The field of dentistry uses up to 10% of the N2O in ambulatory clinics.2
2. The food industry uses approximately 5% to 8% of the N2O manufactured. N2O acts as a propellant for dairy products such as whipped cream.2 Nitrous oxide is dissolved within the cream until it vaporizes with ambient air when expelled from the can. Nitrous oxide produces a whipped cream that is four times the volume of the liquid. Nitrous oxide is lipophilic, thus inhibiting degradation of the fatty cream, whereas if pressurized air was used, the oxygen would cause the cream to become rancid. Nitrous oxide has also been used to displace the oxygen in packages of foods such as potato chips to prolong freshness and prevent crushing.
3. The remaining users of nitrous oxide include the chemical industry, in which nitrous oxide is used in the production of sodium azide, the explosive agent that inflates an automobile air bag.
4. N2O is used to increase engine performance in the racing industry (i.e., cars, motorcycles, boats). An N2O system is now available for personal vehicles as well.
5. N2O is also used in the information technology industry, where it oxidizes chemicals during the manufacture of computer chips. This use requires a 100% pure product.2
6. In a hybrid rocket engine, nitrous oxide has been used as the oxidizer in combination with a fuel source. It has also been used in amateur and high-power rocketry.
C. Manufacturers of N2O
1. The industrial gas industry has seen many changes in the last century. More gas is being required to keep up with technological demands, more countries are using gas for various purposes, advancing healthcare practices and the increasing aging population necessitate additional gas, and new industry lends itself to new applications of gas use.3 The participants in the industrial gas manufacturing world have changed over the years. Mergers and acquisitions have occurred. Several major companies have longstanding history dating back to the early 1900s. No U.S. manufacturers of the small cartridges commonly referred to as “whippets” for whipping cream dispensers are known.
2. The healthcare industry has been slated to be the fastest growing aspect of specialty gases.3 Millions of pounds of N2O are produced annually.
D. Product Expense
1. The entire manufacturing process is very inexpensive and provides an excellent product that is affordable both for the healthcare field and for industry.
2. The cost analysis of N2O given in Appendix I does not include the costs of filling, storing, maintaining, or delivering the cylinders. These costs vary geographically and depend on the volume requested by the customer, the tank size, and the distance from the manufacturing site to the customer.
2 Regulation and Control of Nitrous Oxide
A. Regulatory Agencies
1. The Food and Drug Administration (FDA) regulates the N2O industry. The FDA has established good manufacturing practices (GMPs) and quality system requirements (QSRs) for companies that produce and package gases. Compliance with these regulations results in a near 100% pure product, which exceeds U.S. Pharmacopeia specifications.
2. In addition, the U.S. Department of Transportation (DOT) oversees the packaging and transport of the gas. N2O is considered a hazardous material because of the pressurization. Therefore, it falls under the restrictions of the DOT Hazardous Materials Regulations. Cylinders, transportation vehicles, and drivers must meet prescribed regulations. Figure 6-2 shows a truck transporting N2O and O2.
3. The Compressed Gas Association (CGA) and the Gases and Welding Distributors Association (GAWDA) have recommended guidelines for nitrous oxide sales and security. These guidelines identify control measures that are intended to minimize the theft of nitrous oxide and promote its legitimate use. The guidelines give directives to producers, commercial carriers, repackagers and distributors, and legitimate users.3–8
B. Storing N2O and O2
1. Hospital settings that use bulk amounts of N2O often store the product in large containers. These containers vary in size but range from a  – to a 14-ton capacity. Obviously, economic cost savings are of greatest advantage in these situations, because the storage, handling, and refilling of cylinders are labor-intensive.
– to a 14-ton capacity. Obviously, economic cost savings are of greatest advantage in these situations, because the storage, handling, and refilling of cylinders are labor-intensive.
2. Most often the gas is placed into smaller storage cylinders by a local distributor for delivery.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses