Breath Malodor
Marc Quirynen, Isabelle Laleman, Jesica Dadamio, Sophie De Geest, Betty Vandekerckhove and Wim Teughels
Semantics and Classification
There are three main categories of halitosis: genuine halitosis, pseudo-halitosis, and halitophobia. Genuine halitosis is the term that is used when the breath malodor really exists and can be diagnosed organoleptically or by measurement of the responsible compounds. A distinction should be made between physiologic and a pathologic halitosis (Figure 52-1). The transient disturbing odor caused by food intake (e.g., garlic, onions, and certain spices), smoking, or medication (e.g., metronidazole) do not reveal a health problem and are common examples of physiologic halitosis. The same is true for “morning” bad breath, as habitually experienced on awakening. This malodor is caused by a decreased salivary flow and increased putrefaction during the night and spontaneously disappears after breakfast or after oral hygiene measures. A persistent breath malodor, by definition, does reflect some pathology (pathologic halitosis). The causes of this will be discussed later in the chapter. When an obvious breath malodor cannot be perceived, but the patient is convinced that he or she suffers from it, this is called pseudo-halitosis. If the patient still believes that there is bad breath after treatment of genuine halitosis or diagnosis of pseudo-halitosis, one considers halitophobia, which is a recognized psychiatric condition.157
Epidemiology
Breath malodor is a common complaint among the general population. It has a significant socioeconomic impact but unfortunately has been neglected until recently by scientists and clinicians, and is still hardly covered in the medical curricula. Halitosis can lead to personal discomfort and social embarrassment, and is still one of the biggest taboos of society. Almost $1 billion a year is spent in the United States on deodorant-type mouth (oral) rinses, mints, and related over-the-counter products to manage bad breath.66 It would be preferable to spend this money on a proper diagnosis and etiologic care instead of short-term and even inefficient masking attempts.
The incidence of halitosis remains poorly documented in most countries. Large-scale studies have been performed for the Japanese,72 Swedish,126 and Chinese65 populations. Smaller investigations in Brazilian,77 Polish,50 and Israeli57,114 habitants have also been reported.
Despite the different approach of each study, if a VSC level of 75 ppb (limit for social acceptance)159 is used as a threshold for halitosis, the prevalence reported in the different populations can be calculated as 23% (late-morning group) and 20% for the Japanese and Chinese studies, respectively. In line with this, prevalences of 27.5% and 29.8% for the Chinese and the Israeli groups, respectively, can be calculated when an organoleptic score of at least 2 is considered as being representative for halitosis. With the same criterion, the calculated prevalence varied between 11.0% and 29.7% for the different age groups in the Polish population. In conclusion, independent of the method used to assess oral malodor and independent of the study population, the results point out that about one in four subjects suffer from persistent bad breath.
From large-scale inventories in multidisciplinary outpatient clinics for breath odor,23,24,99 no gender predominance seems to exist for bad breath, although other studies indicate a higher prevalence in women.74,116 It has also been observed that women seek treatment more often than men.72 Age can range from 5 years to more than 80 years. No association was found between increased age and oral malodor.23,24 Most of the patients had been complaining about breath malodor for several years before seeking proper advice (recent report of the department involving 2000 patients).99
Etiology
In the vast majority, breath malodor originates from the oral cavity. Gingivitis, periodontitis, and especially tongue coating are the predominant causative factors.24,89,90,158,159
A recent large-scale study including 2000 patients with halitosis complaints showed that for those where bad breath could be objectively detected, the cause of it was mostly found within the oral cavity (90%). Tongue coating (51%), gingivitis/periodontitis (13%), or a combination (22%) accounted for the majority of the cases.99 Because a large part of the population has a tongue coating or gingivitis/periodontitis, there is a risk that an intraoral condition is too easily considered as the cause while more important pathologies are overlooked. Indeed, for a minority of patients (4% in the same recent study), extraoral causes could be identified, including ear-nose-throat (ENT) pathologies, systemic diseases (e.g., diabetes), metabolic or hormonal changes, hepatic or renal insufficiency, bronchial and pulmonary diseases, or gastroenterologic pathologies.24,79,94,145
In general, one can identify two pathways for bad breath. The first one involves an increase of certain metabolites in the blood circulation (e.g., due to a systemic disease), which will escape via the alveoli of the lungs during breathing (blood-breath exchange) and it is commonly referred as “extraoral halitosis.” The second pathway (intraoral halitosis) involves an increase of either the bacterial load or the amount of substrate for these bacteria at one of the lining surfaces of the oropharyngeal cavity, the respiratory tract, or the esophagus. All types of infections, ulcerations, or tumors at one of the previously mentioned areas can thus lead to bad breath. Studies also suggest that oral malodor is associated with the total bacterial load of anaerobic bacteria in both saliva and tongue coating.98 The most commonly involved bacteria are Porphyromonas gingivalis, Prevotella intermedia/nigrescens, Aggregatibacter actinomycetemcomitans (previously Actinobacillus actinomycetemcomitans), Campylobacter rectus, Fusobacterium nucleatum, Peptostreptococcus micros, Tannerella forsythia, Eubacterium spp, and spirochetes. A study conducted by Niles and Gaffar made clear that these gram-negative species in particular cause an unpleasant smell by the production of sulfur compounds.80 However, because of the large species diversity found in patients with halitosis, it can be suggested that breath malodor is the result of complex interactions between several bacterial species. A recent study indicates that some gram-positive microorganisms, such as Streptococcus salivarius, also contribute to oral malodor production by deglycosylating salivary glycoproteins, thus exposing their protein core to further degradation by gram-negative microorganisms.130
Recently, the presence of Solobacterium moorei, a gram-positive bacterium, has also been linked to oral malodor.45,44,53
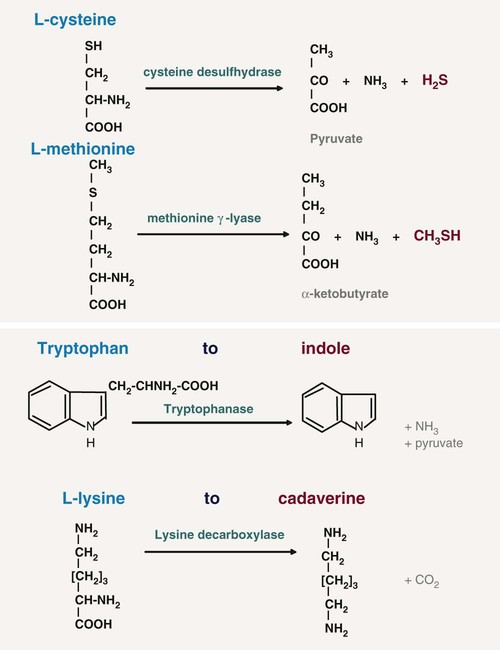
For oral malodor, the unpleasant smell of the breath mainly originates from volatile sulphur compounds (VSCs), especially hydrogen sulfide (H2S), methylmercaptan (CH3SH), and (less significantly) dimethyl sulfide [(CH3)2S], as first discovered by Tonzetich.138 However, in certain conditions (e.g., when the saliva dries out on the mucosal surfaces), other compounds in mouth air may also play a role such as diamines (e.g., putrescine, cadaverine), indole, skatole, and volatile organic acids like butyric or propionic acid.39 Most of these compounds result from the proteolytic degradation by oral microorganisms of peptides present in saliva (sulfur-containing or non–sulfur-containing amino acids) (Figure 52-2), shed epithelium, food debris, gingival crevicular fluid (GCF), interdental plaque, postnasal drip, and blood. In particular, gram-negative, anaerobic bacteria possess such proteolytic activity.
For the extraoral causes of halitosis, other compounds besides the VSCs may be involved. Bad-smelling metabolites can be formed/absorbed at any place in the body (e.g., the liver, the gut) and transported by the bloodstream to the lungs. Exhalation of these volatiles in the alveolar air then causes halitosis, at least when the concentrations of the bad-smelling metabolites are sufficiently high. The crevicular fluid reflects the circulating molecules in the blood and can thus also play a relevant role but due to the small amount probably not a very dominant one. The extraoral causes are much more difficult to detect, although they can sometimes be recognized by a typical odor. Uncontrolled diabetes mellitus can be associated with a sweet odor of ketones, liver disease can be revealed by a sulfur odor, and kidney failure can be characterized by a fishy odor because of the presence of dimethylamine and trimethylamine.94
In a special patient category, subjects imagine they have breath malodor; this is called imaginary breath odor or halitophobia.86 The latter has been associated with obsessive-compulsive disorders and hypochondria. Well-established personality disorder questionnaires (e.g., Symptom Checklist 90) allow the clinician to assess the patient’s tendency for illusional breath malodor.25,26,28 The presence of a psychologist or psychiatrist at the malodor consultation can be especially helpful for such patients. Because of the complexity of this pathology, a malodor consultation is thus preferably multidisciplinary, combining the knowledge of a periodontologist or dentist, an ENT specialist, an internist (if necessary), and a psychologist or psychiatrist. In a recent study of 2000 patients, 16% were diagnosed with pseudo-halitosis or halitophobia.99
Intraoral Causes
Tongue and Tongue Coating.
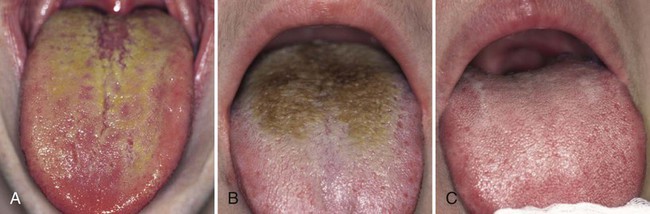
The dorsal tongue mucosa, with an area of 25 cm2, shows a very irregular surface topography.16,122 The posterior part exhibits a number of oval cryptolymphatic units, which roughen the surface of this area. The anterior part is even rougher because of the high number of papillae: the filiform papillae with a core of 0.5 mm in length, a central crater and uplifted borders; the fungiform papillae, 0.5 to 0.8 mm in length; the foliate papillae, located at the edge of the tongue, separated by deep folds; and the vallate papillae, 1 mm in height and 2 to 3 mm in diameter. These innumerable depressions in the tongue surface are ideal niches for bacterial adhesion and growth, sheltered from cleaning actions.22,158 Moreover, desquamated cells and food remnants also remain trapped in these retention sites and consequently can be putrefied by the bacteria.5 A fissurated tongue (deep fissures on dorsum, also called scrotal tongue or lingua plicata) and a hairy tongue (lingua villosa) have an even rougher surface (Figure 52-3).
The accumulation of food remnants intermingled with exfoliated cells and bacteria forms a coating on the tongue dorsum. The latter cannot be easily removed because of the retention offered by the irregular surface of the tongue dorsum (see Figure 52-3). As such, the two factors essential for putrefaction are united. Several investigators have identified the dorsal posterior surface of the tongue as the primary source of breath malodor.5,15,22,111 Indeed, high correlations have been reported between tongue coating and odor formation.5,18,72,158
Both in healthy individuals and periodontitis patients with or without complaints of oral halitosis, a significant positive correlation was found between the presence or amount of tongue coating and levels of VSCs72,100 and/or organoleptic scores of the mouth odor.22 In a group of 2000 patients visiting a multidisciplinary halitosis clinic,99,150 significant correlations were found between the organoleptic scores and tongue coating (R = 0.52; p < 0.001). In another study,84 it was also observed that the amount of tongue coating was significantly greater in the halitosis-positive group compared to the halitosis-negative group.
Morita and Wang74 found that the volume of tongue coating and the percentile of sites with bleeding on probing, were significantly associated with oral malodor.74 In 1992, Yaegaki and Sanada158,159 demonstrated that, even in patients with periodontal disease, 60% of the VSCs were produced from the tongue surface.158,159
Recent research showed that the strongest determinant for the presence of tongue coating is suboptimal oral hygiene. Other influencing factors were: periodontal status, presence of a denture, smoking, and dietary habits.149
Periodontal Infections.
A relationship between periodontitis and oral malodor has been shown. However, not all patients with gingivitis and/or periodontitis complain about bad breath, and there is some disagreement in the literature as to what extent oral malodor and periodontal disease are related.5,112,127 Bacteria associated with gingivitis and periodontitis are indeed able to produce VSCs.55,78,80,89,90,138
Several studies have shown that the VSC levels in the mouth correlate positively with the depth of periodontal pockets (the deeper the pocket, the more bacteria, particularly anaerobic species) and that the amount of VSCs in breath increases with the number, depth, and bleeding tendency of the periodontal pockets.15,88,99,159 It is important to realize that VSCs aggravate the periodontitis process by, for example, increasing the permeability of the pocket and mucosal epithelium and therefore exposing the underlying connective tissues of the periodontium to bacterial metabolites. Moreover, methylmercaptan enhances interstitial collagenase production, interleukin-1 (IL-1) production by mononuclear cells, and cathepsin B production, thus further mediating connective tissue breakdown.63,104 It was also shown that human gingival fibroblasts developed an affected cytoskeleton when exposed to methylmercaptan.7,104 The same gas alters cell proliferation and migration. VSCs are also known to impede wound healing. Thus, when periodontal surgery is planned, especially the insertion of implants, clinicians should recognize this pathologic role of VSCs.
Some studies, however, have shown that when the presence of tongue coating is taken into account, the correlation between periodontitis and oral malodor is much lower, indicating that tongue coating remains a key factor for halitosis. The prevalence of tongue coating is six times higher in patients with periodontitis, and the same bacterial species associated with periodontal disease can also be found in large numbers on the dorsum of the tongue, particularly when tongue coating is present.158 The reported association between periodontitis and oral malodor may thus primarily be due to the effects of periodontal disease on tongue coating. And may explain why other articles did not find a correlation.5,127
Other relevant malodorous pathologic manifestations of the periodontium are pericoronitis (the soft tissue “cap” being retentive for microorganisms and debris), major recurrent oral ulcerations, herpetic gingivitis, and necrotizing gingivitis/periodontitis. Microbiologic observations indicate that ulcers infected with gram-negative anaerobes (i.e., Prevotella and Porphyromonas species) are significantly more malodorous than noninfected ulcers.6
Dry Mouth.
Saliva has an important cleaning function in the oral cavity. Patients with xerostomia often present with large amounts of plaque on teeth and an extensive tongue coating. The increased microbial load and the escape of VSCs when saliva is drying up explain the strong breath malodor.55 Several studies link stress with VSC levels, but it is not clear whether this can simply be explained by a reduction of salivary flow.61,96 Other causes of xerostomia are medication,73 alcohol abuse,34 Sjögren syndrome (a common autoimmune rheumatic disease),68 and diabetes.151
Extraoral Causes
Ear-Nose-Throat.
During chronic or purulent tonsillitis, the deep crypts of the tonsils accumulate debris and bacteria, especially periopathogens, resulting in putrefaction. In the crypts, even calculus (e.g., subgingivally) can be formed (tonsilloliths or tonsil stones). Other examples include acute pharyngitis (viral or bacterial) and postnasal drip. The latter is a rather common condition, which is perceived by patients as a liquid flow in the throat, originating from the nasal cavity.111 It is often associated with chronic sinusitis or regurgitation esophagitis, in which the acidic content of the stomach reaches the nasopharynx and causes mucositis. Ozena (caused by Klebsiella ozaenae) is a rare atrophic condition of the nasal mucosa, with the appearance of crusts that causes a very strong breath malodor. Finally, a foreign body in a nasal or sinus cavity can cause local irritation, ulceration, and subsequent putrefaction (e.g., children and mentally handicapped persons tend to put objects such as peas or wet paper in the nose).
Bronchi and Lungs.
Pulmonary causes include chronic bronchitis, bronchiectasis (infection of standing mucus secretion in cystic dilations through walls of bronchioles), pneumonia, pulmonary abscesses, bronchial carcinoma, and carcinoma of the lung.67,69 The relevance of an early diagnosis is evident.
Gastrointestinal Tract.
In contrast to the common public opinion, even among medical physicians, gastrointestinal pathologies are rarely responsible for bad breath.83,137 The following pathologies might be responsible for less than 1% of malodor cases:
• A Zenker diverticulum (a hernia in the esophageal wall, allowing accumulation of food and debris and thus putrefaction) can cause a significant breath odor because it is not separated from the oral cavity by any sphincter.17
• A gastric diaphragmatic hernia (the fundus of the stomach protrudes through the diaphragm with relative sphincter insufficiency, allowing gases to escape or contents to flow back in the esophagus) can cause reflux of the gastric contents up to the oropharynx. This is sometimes combined with ructus, in which air from the stomach suddenly regurgitates.
• Regurgitation esophagitis (ulceration of the mucosal lining of the esophagus by acidic stomach contents flowing back because of an improper function of the sphincter).54
• Intestinal gas production: Some gases (e.g., dimethyl sulfide) are absorbed but not metabolized by the intestinal endothelium and thus transported by the blood. These gases can then be exhaled through the lungs.132
There is some disagreement in the literature whether Helicobacter pylori infection is associated with halitosis.52,75 In a recent paper, H. pylori was shown to produce hydrogen sulfide and methylmercaptan, which suggests that this microorganism can contribute to the development of halitosis.64 Tangerman et al137 found no association between halitosis and H. pylori infection.137
Liver.
Patients with various degrees of hepatocellular failure and/or portosystemic shunting of blood may acquire a sweet, musty, or even slightly fecal aroma of the breath, termed fetor hepaticus, which has been mainly attributed to the accumulation of dimethyl sulfide.10,135,143 Moreover, if the metabolizing function of the liver fails, the concentration of certain metabolites, normally processed in the liver, will increase and they will enter the systemic circulation again and will be exhaled.18,141
Systemic Metabolic Disorders.
Uncontrolled diabetes mellitus results in the accumulation of ketones, which have a sweet smell, like the odor of rotten apples. Insulin resistance leads to an increase of triglycerides and free fatty acids and ketones (such as acetone, acetoacetate, and hydroxybutyrate) are formed during lipolysis.131
Trimethylaminuria.
Trimethylaminuria is a hereditary metabolic disorder that leads to a typical fishy odor of the breath, urine, sweat, and other bodily secretions. Trimethylaminuria is an enzymatic defect that prevents the transformation of trimethylamine to trimethylaminoxide, resulting in abnormal amounts of this molecule. The prevalence is unknown but approaches 1% in the United Kingdom.71
Hormonal Causes.
At certain moments during the menstrual cycle, a typical breath odor can develop; partners are often well aware of this odor. Evidence also indicates that VSC levels in the expired air are increased twofold to fourfold around the day of ovulation and in the perimenstrual period. Increases in VSCs are smaller in midfollicular phases.94
Fundamentals of Malodor Detection
The breath of a person contains up to 150 different molecules.94,92,142,143,144 The characteristics of the expired molecules determine whether we can smell them or not. Some gases can cause a striking odor at very low concentrations, whereas others need to be present in much higher quantities. The perception of the molecules depends on the following factors40:
1. The odor itself (olfactory response) can be pleasant, unpleasant, or even repulsive.
2. Each particular molecule has its specific concentration before it can be detected (threshold concentration).
3. The odor power is the extent of concentration that is necessary to increase the odor score with one unit.
4. The volatility of the compound: Malodorous molecules only express themselves when they become volatile.
5. The substantivity: The capacity of the molecule to stay present and thus to remain the cause of smell.
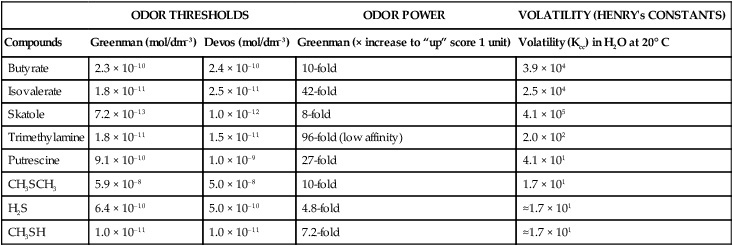
Table 52-1 provides an overview of these essential characteristics for the key malodorous compounds, clearly highlighting large interproduct variations. The odor power is the strongest for hydrogen sulfide and methylmercaptan; if the concentration of these products increases fivefold to tenfold, the odor will receive a higher organoleptic rating. For some other compounds, increases of 25 to 100 times are needed to reach a similar effect. Skatole and methylmercaptan are detected at the lowest concentrations (low threshold). The three sulfur molecules have the lowest volatility (i.e., will escape the liquid phase first).40
TABLE 52-1
Odor Threshold and Odor Power of Key Malodorous
| ODOR THRESHOLDS | ODOR POWER | VOLATILITY (HENRY’s CONSTANTS) | ||
| Compounds | Greenman (mol/dm–3) | Devos (mol/dm–3) | Greenman (× increase to “up” score 1 unit) | Volatility (Kcc) in H2O at 20° C |
| Butyrate | 2.3 × 10–10 | 2.4 × 10–10 | 10-fold | 3.9 × 104 |
| Isovalerate | 1.8 × 10–11 | 2.5 × 10–11 | 42-fold | 2.5 × 104 |
| Skatole | 7.2 × 10–13 | 1.0 × 10–12 | 8-fold | 4.1 × 105 |
| Trimethylamine | 1.8 × 10–11 | 1.5 × 10–11 | 96-fold (low affinity) | 2.0 × 102 |
| Putrescine | 9.1 × 10–10 | 1.0 × 10–9 | 27-fold | 4.1 × 101 |
| CH3SCH3 | 5.9 × 10–8 | 5.0 × 10–8 | 10-fold | 1.7 × 101 |
| H2S | 6.4 × 10–10 | 5.0 × 10–10 | 4.8-fold | ≈1.7 × 101 |
| CH3SH | 1.0 × 10–11 | 1.0 × 10–11 | 7.2-fold | ≈1.7 × 101 |
Greenman J, Duffield J, Spencer P, et al: J Dent Res 83:81, 2004.
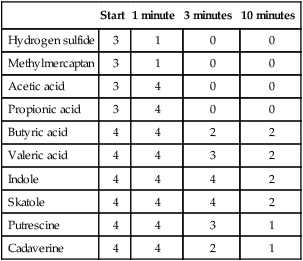
In a study by Kleinberg and Codipilly, aqueous solutions of oral odoriferous volatiles were placed on the skin of the back of the hand.55 Afterwards, odor scores were given (organoleptic score, cfr. supra). The results are shown in Table 52-2. All metabolites caused an explicit odor, which decreased in intensity over time. Some molecules disappeared very fast (e.g., hydrogen sulfide and methylmercaptan), whereas others produced a bad smell for a longer period of time (e.g., indole and skatole, for 10 minutes and longer).
TABLE 52-2
Odor Scores at the Back of the Hand
| Start | 1 minute | 3 minutes | 10 minutes | |
| Hydrogen sulfide | 3 | 1 | 0 | 0 |
| Methylmercaptan | 3 | 1 | 0 | 0 |
| Acetic acid | 3 | 4 | 0 | 0 |
| Propionic acid | 3 | 4 | 0 | 0 |
| Butyric acid | 4 | 4 | 2 | 2 |
| Valeric acid | 4 | 4 | 3 | 2 |
| Indole | 4 | 4 | 4 | 2 |
| Skatole | 4 | 4 | 4 | 2 |
| Putrescine | 4 | 4 | 3 | 1 |
| Cadaverine | 4 | 4 | 2 | 1 |
The olfactory response, rated by an organoleptic scale, follows an exponential curve when correlated with the concentration of different gases. In other words, when the concentrations of the molecules were compared to their organoleptic outcome, an optimal fit was only obtained when the concentrations of the gases were transformed to log values. The latter implies that all types of breath “measurements” (gas chromatography [GC], portable breath analyzers [e.g., Halimeter]) require log transformation to be comparable with organoleptic scores.40
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses