5
The Immune System
The immune system is a wonderful and complex defensive system designed to recognize foreign material and render that material harmless. This obligation, as you will see, is more involved and demanding than national defense.
The defensive network of the immune system has several “branches” of armed services with different styles and even territories of operations. Like most defensive systems, the branches support one another.
The enemies to the system can be virtually anything that is not the system itself. The enemy can come in endless disguises, and to make things more dangerous, even parts of the system itself can be identified as “enemy.” The code word for enemy is antigen. The host defense system doesn’t care what disguise the enemy comes in, because the “giveaway” is the antigen molecule that is attached.
To be effective, a defensive system has to be able to assemble raw recruits, train them to quickly recognize the enemy from the allies, engage in battle with effective weapons, and remain watchful and ready to mobilize at a moment s notice during the lulls.
What follows is a brief description of the opposing sides. Since we don’t care what the enemy actually is (we also have an excellent propaganda machine), we will describe antigen with group characteristics. Next, we will describe the good guys (defenders) with particulars. These are the special forces (lymphocytes) and support units (antigen-presenting cells). After you meet the gang, we’ll take you to war!
The defenders
Here are the defenders:
- Lymphocytes—the fighting units of the immune system
- Antigen-presenting cells—the tactical support units for the lymphocytes
- Macrophages—produce cytokines and lyse tumor cells
- Cytokines—regulatory proteins produced by T cells and other cells of the immune system
- Antibodies—weapon support units for the lymphocytes
Lymphocytes
Basically, there are three types of lymphocytes: T cells, B cells, and natural killer (NK) cells.
Lymphocytes are found throughout the body in lymph nodes, the spleen, the bloodstream, lymphatics, Peyer’s patches, tissues, and secretions.
T cells and B cells are programmed to respond to specific antigens, whereas NK cells do not require prior sensitization to perform their functions. The progeny of activated B cells are plasma cells, which secrete antibodies. NK cells are capable of killing tumor cells, as well as virus-infected and other cells.
T cells
T cells arise from stem cells in the bone marrow (Fig 5-1). After pre-T cells leave the bone marrow, they undergo further maturation in the thymus gland. Thus, T cells are sometimes referred to as thymus-dependent lymphocytes.
During the final stage of T-cell development, the cells migrate from the thymus to the blood and lymphatic system. The preponderance of circulating lymphocytes are T cells, and they recirculate constantly from the bloodstream to the lymphatics. From the bloodstream they enter and patrol the tissues.
Mature T cells are located in the peripheral lymphoid tissues, particularly in the paracortical region of the lymph nodes (Fig 5-2) and in the periarteriolar portions of the white pulp of the spleen.
There are two basic subsets of T cells, those that express surface CD4 antigens and those that express CD8 antigens. (Note: CD stands for cluster designation.)
CD4+ T cells are helper cells that secrete soluble factors (cytokines) that influence virtually all of the cells of the immune system, including other T cells, B cells, macrophages, and NK cells. There are two subsets of these cells, Th1 and Th2 cells, to be discussed later.
CD8+ T cells are either cytotoxic or suppressor cells. Suppressor cells inhibit the immune response to a specific antigen or immune cell; they do not suppress responses to other antigenic determinants. Thus they can serve in a regulatory capacity over the immune system. Here are your MPs!
Figure 5-3 provides an example of how helper and suppressor T cells influence a B cell that has bound specific antigen to its receptors. The T-helper cell promotes proliferation and differentiation, whereas the suppressor cell has blocked proliferation.
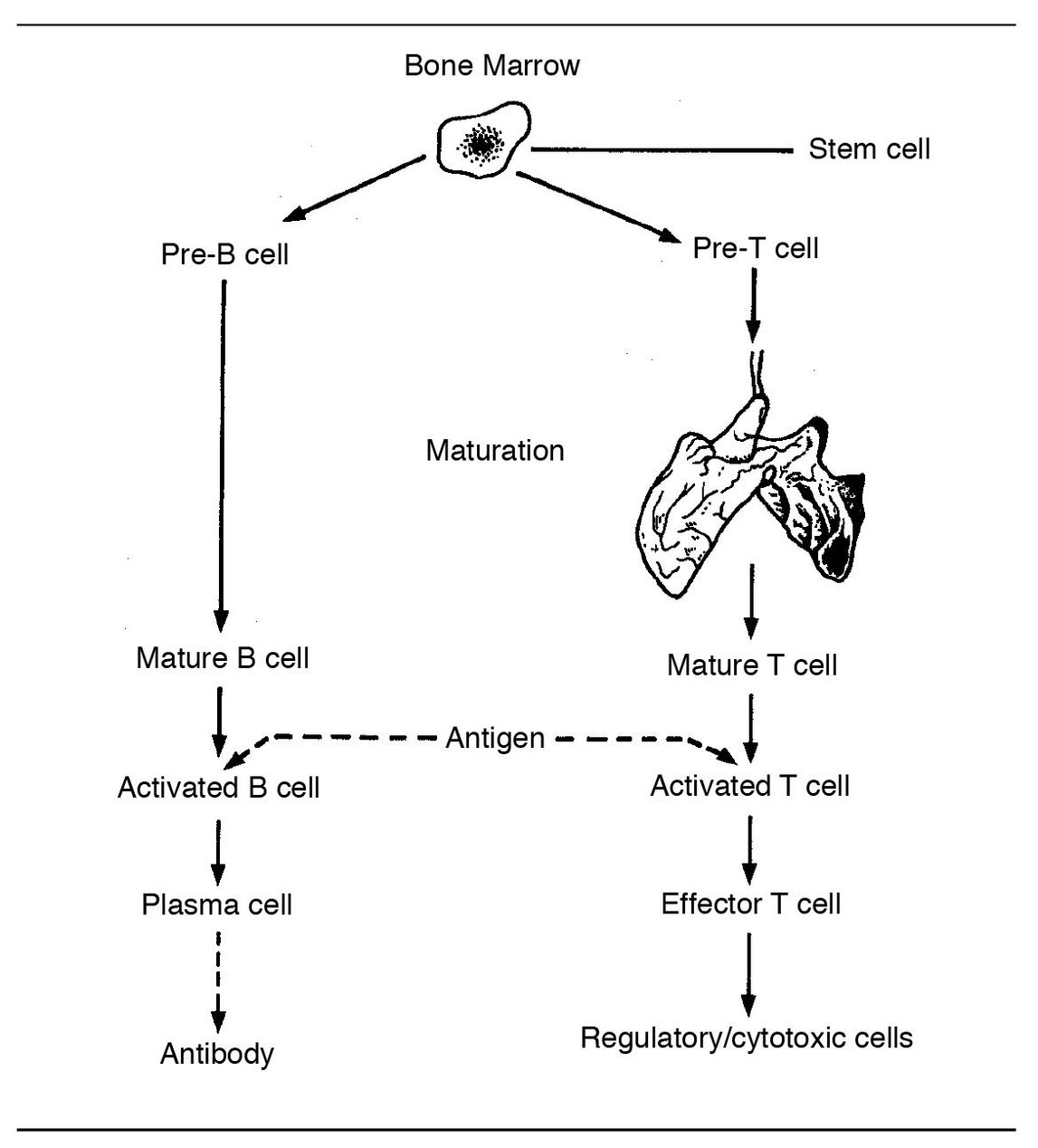
Fig 5-1 Immune response.
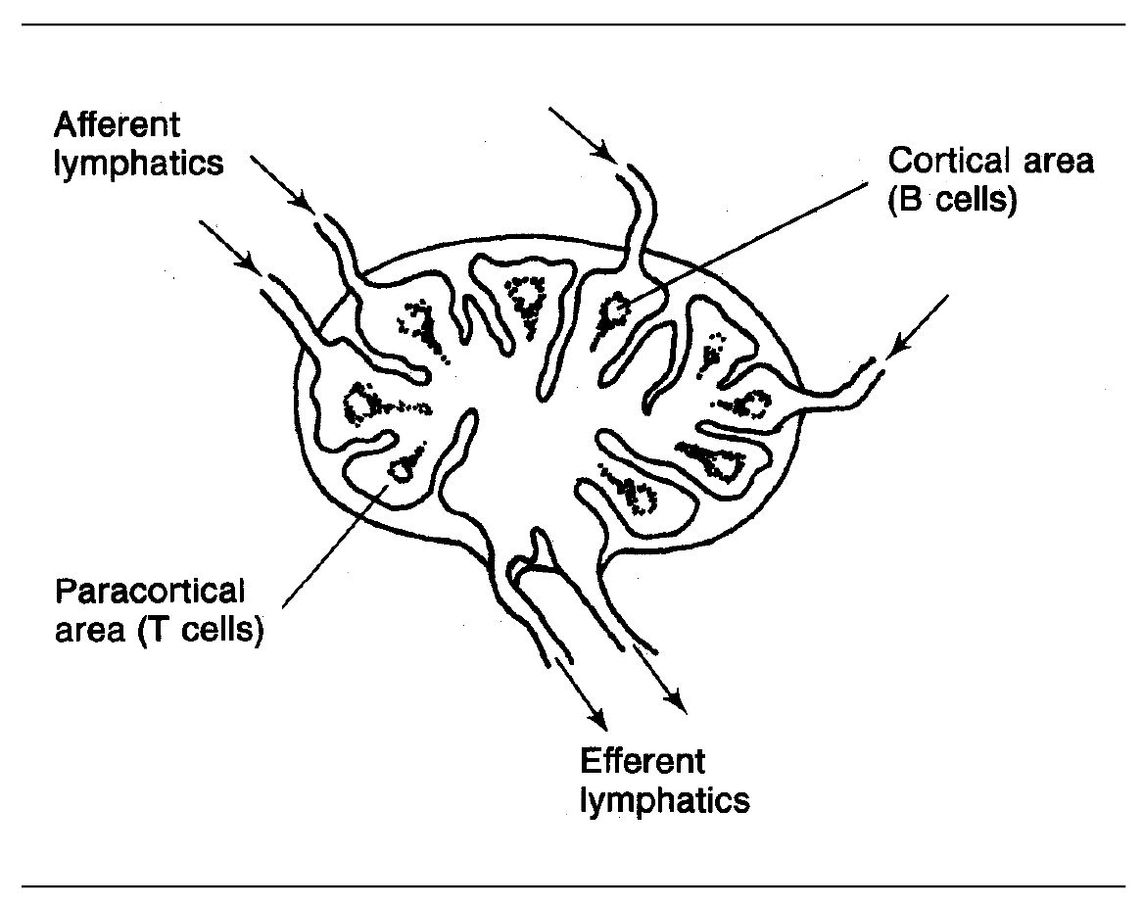
Fig 5-2 Lymph node.
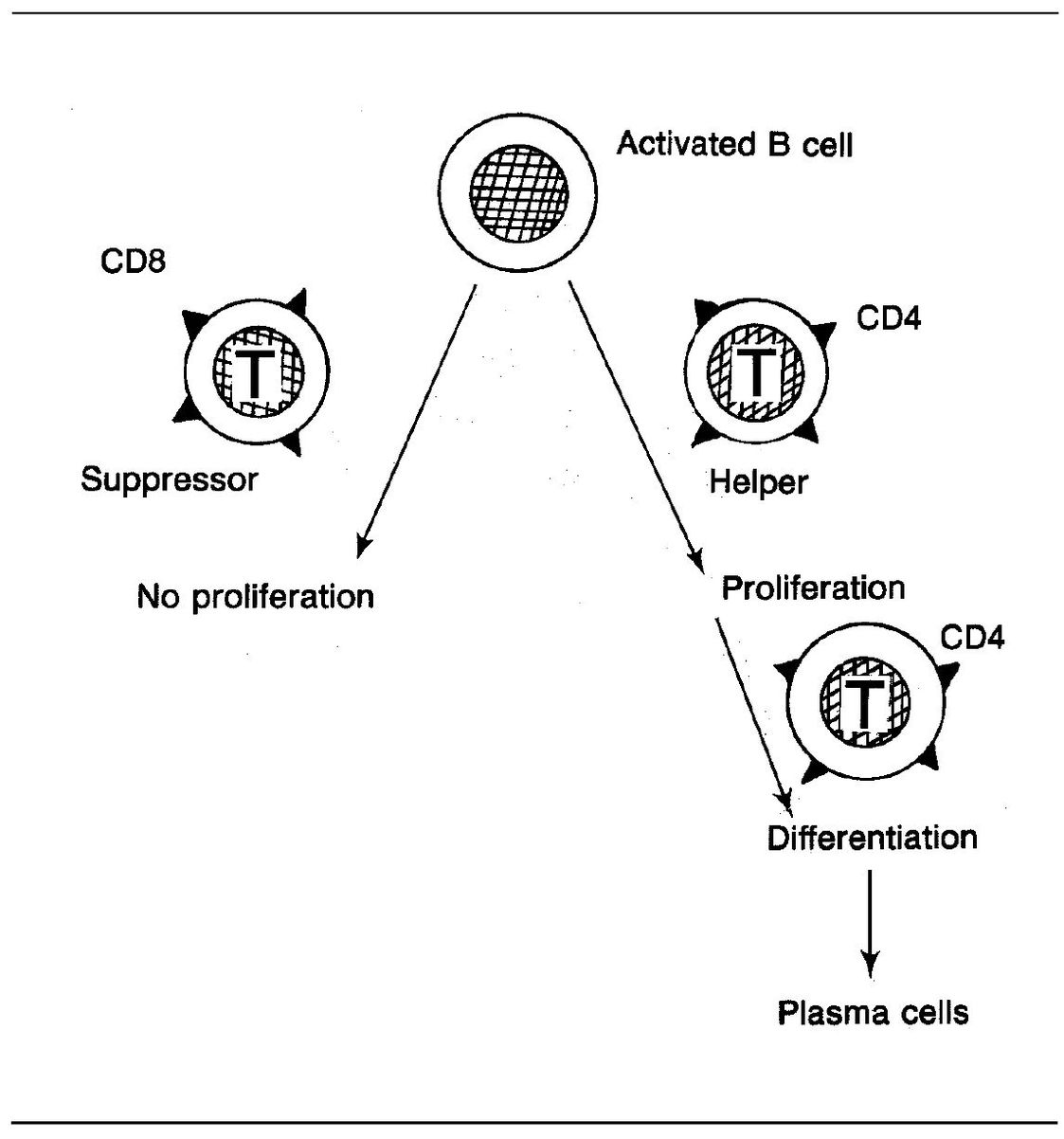
Fig 5-3 T-cell regulatory functions.
Cytotoxic T lymphocytes (CTLs) are able to kill antigen-bearing cells such as tumor cells or virus-infected cells. These CD8+ T cells kill target cells by direct contact, that is, killing only takes place when the CTL and its target cell come together so that their cell membranes are in direct contact (Fig 5-4).
The exact mechanism of killing is not known, but it may involve the release of perforin, a Ca++-dependent protein, from secretory granules in CTLs. Perforin is similar to C9 of the complement system in that it can insert itself into the membrane of a target cell and produce cell lysis. CTLs also release serine hydrolases. The role of these proteolytic hydrolases is unclear, but specific hydrolase inhibitors block the cyotoxicity of CTLs.
CTLs can also elaborate tumor necrosis factor-beta (TNF-β), which has the ability to kill certain cells by a process known as apoptosis (programmed cell death), in which there is a very rapid fragmentation of the target cell nucleus.
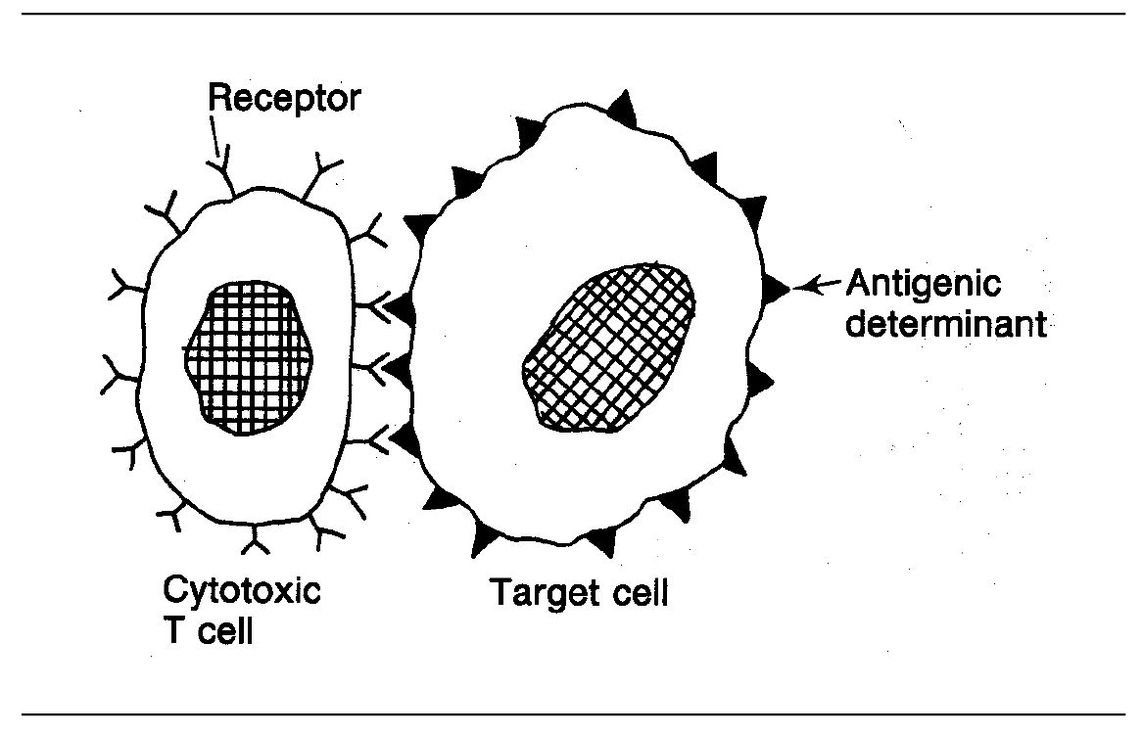
Fig 5-4 Cytotoxic T lymphocyte.
CD4 is present on 60% of peripheral T cells, whereas CD8 is present on 30%. The ratio of CD4+ to CD8+ lymphocytes in the blood of healthy individuals is approximately 2 to 1.
T-CD4+-helper cell subsets
Two subsets have been identified, Th1 and Th2.
Th1 cells are primarily involved in macrophage-dependent immune responses and synthesize and secrete interleukin-2 (IL-2) and interferon-gamma (INF-γ), both of which are involved in delayed hypersensitivity. In addition, these lymphocytes regulate the production of opsonizing antibodies.
Th2 cells facilitate the synthesis of other types of antibodies. They synthesize and secrete IL-4 and IL-5.
The equilibrium between Th1 and Th2 cells is regulated by the balance between interleukin-12 (IL-12) and interleukin-4 (IL-4) early in the immune response. IL-12 promotes the generation of Th1 cells, whereas IL-4 promotes the production of Th2 cells.
Thus, there are separate subpopulations of T cells, which:
- Mediate positive aspects (CD4+ T cells)
- Mediate negative aspects (CD8+ T-suppressor lymphocytes)
- Become effector cells and produce cytokines
- Directly kill other cells
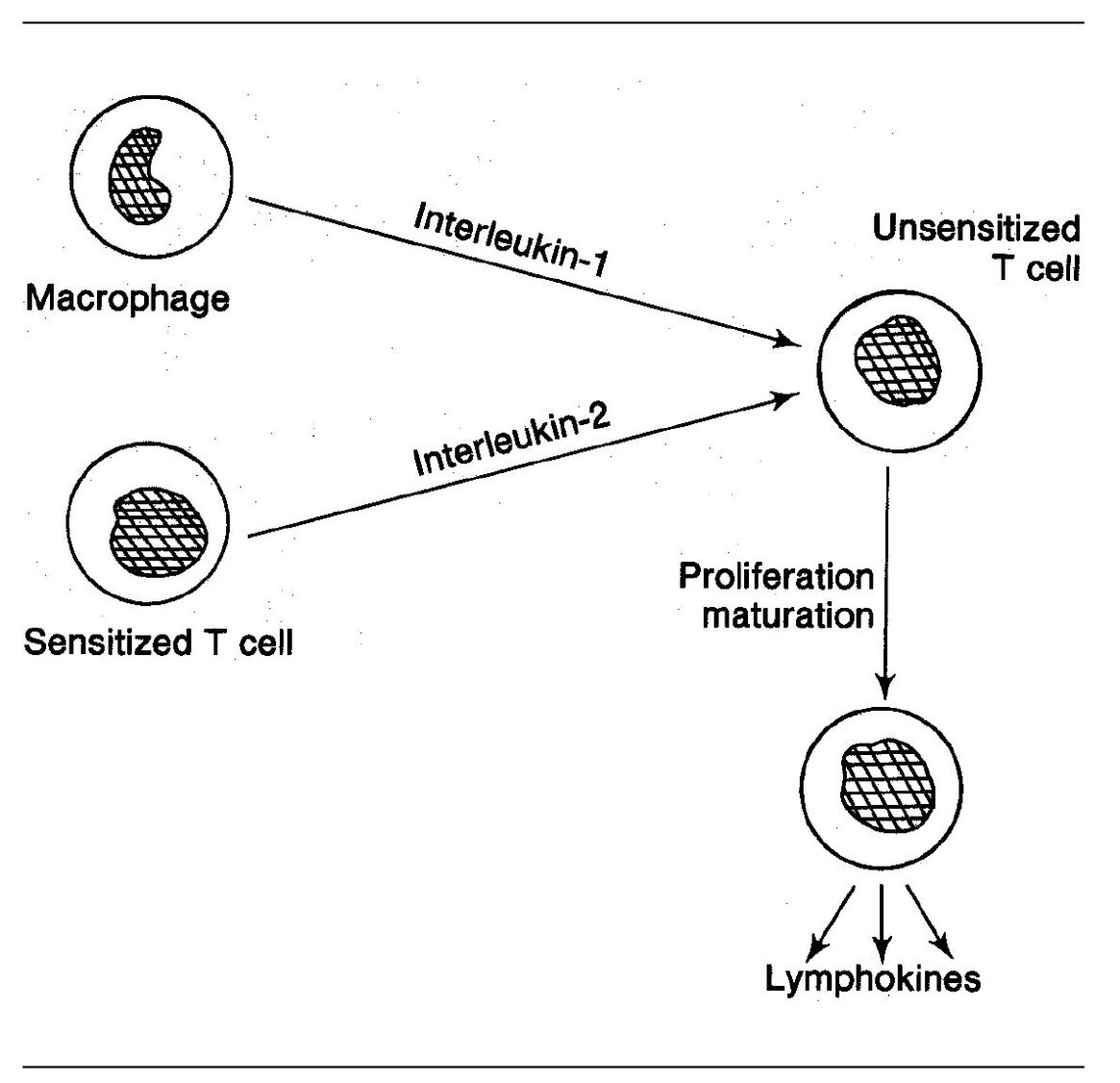
Fig 5-5 T-cell recruitment.
Cell recruitment
T cells give rise to specific sensitized lymphocytes. Some of these sensitized cells have the ability to activate nonsensitized T cells. This activation is associated with a very important phenomenon known as cell recruitment. This involves the following:
- Sensitized T cells reach the site of antigen deposition.
- Upon contacting the antigen, they release cytokines (see page 88).
- Among other things, cytokines have the ability to make nonsensitized T cells behave as though they are sensitized, that is, they too will release cytokines and do other things sensitized lymphocytes can do (even though they are not precommitted to respond to the same antigen). Interleukin-1 and interleukin-2 facilitate this process (Fig 5-5).
- T-cell cytokines also recruit other cells, notably macrophages and PMNs.
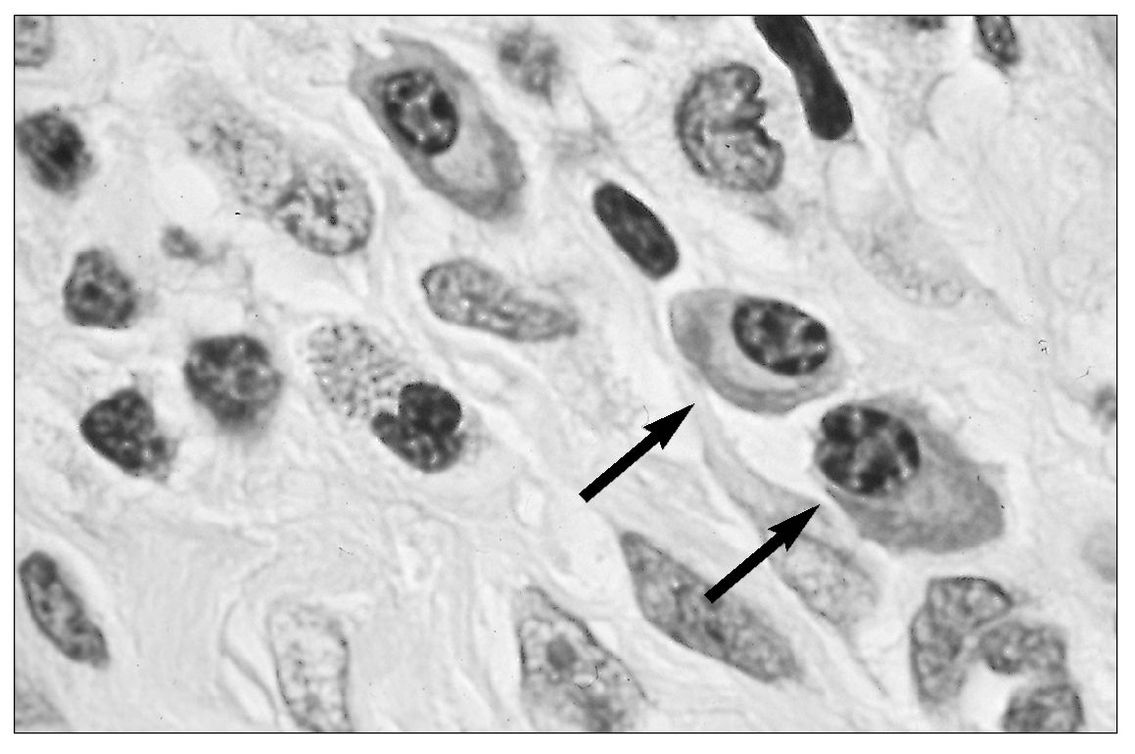
Fig 5-6 Plasma cells (arrows) in an inflammatory cell infiltrate.
B cells
B cells are present in blood (10% to 20% of the lymphocytes in circulating blood), lymphoid tissue, and bone marrow. In immune reactions, they give rise to antibody-producing plasma cells (Fig 5-6).
B-cell precursors migrate from the embryonic yolk sac to the fetal liver and later to the bone marrow, where they multiply and diversify into numerous clones (see Fig 5-1). Immature B cells express surface monomeric lgM; surface IgD appears when the B cell is mature. Differentiated B cells accumulate in or near the germinal centers in the follicles of extrathymic lymphoid tissues, that is, lymph nodes (see Fig 5-2) and spleen.
B-cell activation generally requires two signals: the binding of antigen to the B cell’s receptors, and stimulation of the B cell by T-cell cytokines. Any given antigen usually activates both T cells and B cells. In many cases, T cells and B cells are functional partners in producing full immunologic reactivity. They do not work alone.
T-helper cells bind to antigen on the surface of a B cell that has already bound itself to the antigen. The helper cell then promotes activation and maturation of the B cell.
Antigens that can evoke an antibody response are referred to as T-independent antigens. These are typically polymeric and large in size with repeating antigenic sequences. At times, these antigens can activate a large number of B cells nonspecifically, a phenomenon referred to as polyclonal B-cell activation. A number of infectious agents are capable of causing this form of activation (eg, Epstein-Barr virus, LPS, and Borrelia burgdorferi [Lyme disease]).
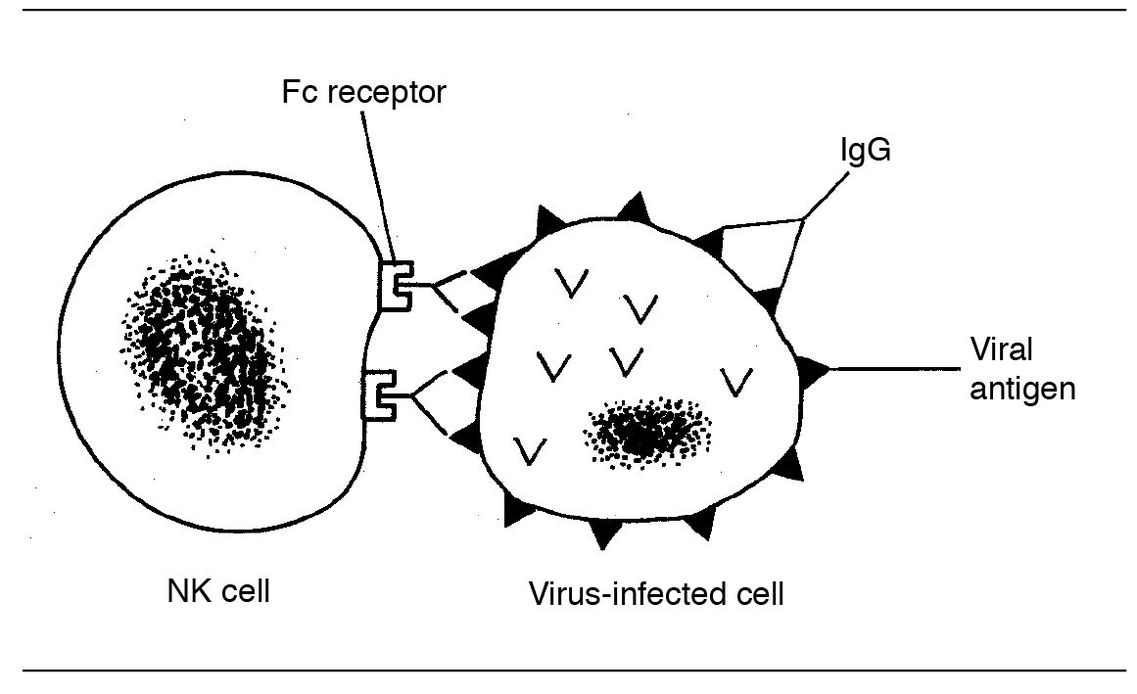
Fig 5-7 NK cell killing.
Natural killer cells
These cells are present in the lymphoid tissues of nonimmu-nized individuals. They are not T cells or B cells, but appear to be another form of lymphocyte sometimes referred to as large granular lymphocytes. They represent about 15% of the total lymphocyte population. Peripheral blood contains about six times as many T cells as NK cells, but NK cells outnumber circulating B cells.
The primary importance of these cells is that they protect against viral infections by lysing virus-infected cells, and against cancer by destroying cells that have undergone malignant transformation. It appears that NK cells recognize sites on high molecular weight glycoproteins that are expressed on the surface of virally infected cells. This enables them to differentiate infected cells from normal cells.
NK cells do not recognize antigen, so they require neither antigen processing nor presentation of antigen for activation. Therefore they can be mobilized quickly, days before a more specific T-cell response can be generated. They can kill various target cells directly, and they are also able to mediate antibody-dependent cellular cytotoxicity. This is because these cells have Fc receptors and can bind to cell-bound antibody via the Fc portion of the antibody (ADCC) (Fig 5-7).
Granules within NK cells (as well as the cytoplasm of CTLs) contain a pore-forming protein, called perforin. Degranulation results in the secretion of perforin into the extracellular space between the NK cell and the target cell. Perforin is then inserted into the plasma membrane of the target cell and produces pores with internal diameters of up to 20 nm in the membrane. This destroys the target cell s permeability barrier. (This is similar to the MAC of complement, discussed earlier. In fact, antibodies against perforin cross react with the MAC of complement.)
Recent experiments suggest that perforin is employed in situations in which there are high local concentrations of interleukin-2, such as cancer and acute viral infections.
NK cells respond rapidly to cytokines such as IL-2 and interferon to generate both a proliferative and cytologic response.
Because NK cells can lyse target cells without any apparent previous sensitization and without involvement of major histocompatibility complex (MHC) antigens, they play a key role in immune surveillance.
You may come across the term lymphokine-activated killer (LAK) cells. These are peripheral blood lymphocytes (PBLs) that have been incubated in culture with high levels of IL-2. They are similar to NK cells but the range of cells they can kill is much greater. Most of these LAK cells are derived from NK cells but some are from CTLs. When injected into the cancer patient from which the PBLs were withdrawn, marked regression of tumors has been reported. However, adverse side effects have limited this form of cancer therapy.
Self-test 5-1
- What is an antigenic determinant?
- The majority of antigens are what kind of molecule?
- What is a hapten?
- What are histocompatibility antigens?
- What is their importance in organ transplantation?
- Where in the lymph nodes and spleen are T cells located? Where are the B cells?
- Where do T cells mature?
- What is the chief difference between B and T lymphocytes and NK cells?
- What cell is the progenitor of plasma cells?
- What is meant by polyclonal B-cell activation?
Answers on page 216.
Antigen-presenting cells
These cells are also known as accessory cells of the immune system.
Antigen-presenting cells (APCs) have an early role in recognizing the enemy before “war is declared.” APCs are non-antigen-specific cells of the immune system. They capture (phagocytize) antigen molecules that invade the body and break them down into smaller fragments.
As antigen enters the body and is encountered by APCs, it is processed and presented in a form that can be recognized by the T-cell receptor (TCR) on the T-helper cell. This initiates an ordered sequence of signals that leads to the activation of T-helper cells, thus triggering an immune response.
Whereas B cells can often be activated by free antigen, T cells are only able to recognize antigen in the form of MHC class II antigen (more on this later in this chapter). These antigen fragments (peptides) are termed antigenic determinants. A synonym for the recognized part of the antigen is the epitope. Within the APC, antigenic determinants combine with a gene product of the MHC. This complex is expressed on the surface of the APC where it can be “presented” (recognized) by the TCR on the T-helper cell.
Cells classified as APCs include dendritic cells (cells having dendritic processes), such as Langerhans’ cells of the epidermis. Macrophages, B cells, and endothelial cells can also serve as APCs.
Dendritic cells are found in most tissues and are particularly numerous in the lymphoid tissues, where there are large numbers of lymphocytes with which to interact. Dendritic cells in lymph nodes are termed follicular dendritic cells (FDCs). FDCs catch and hold bacterial or viral antigens as a mesh screen catches lint. For months or even years, memory cells of the immune system refer back to these antigens for information on how to combat these bacteria or viruses.
A very important function of APCs is the production of interleukin-12. This will be discussed later in this chapter.
Unlike macrophages, Langerhans cells and dendritic cells are only weakly phagocytic and do not engage in microbicidal activities.
Besides being APCs, macrophages produce a variety of cytokines, such as interleukin-1 and tumor necrosis factor. By secreting free radicals and proteolytic enzymes they can kill tumor cells. They also play a key role in cell-mediated hypersensitivity (see chapter 6).
Macrophages are stimulated by cytokines produced by the Th1 subset of T cells.
Cytokines
Cytokines were called lymphokines when it was thought they were only secreted by T cells. It is now known that other cells, such as macrophages, secrete them.
Cytokines are soluble polypeptide products of cells of the immune system. Those produced by macrophages are sometimes termed monokines. A few facts about cytokines:
- They can modify the behavior of other cells as well as the cells that produce them. These are regulatory cytokines. Others induce differentiation of cells such as T cells and B cells.
- Some can gain access to the general circulation and produce systemic effects such as fever, ACTH production, and release of neutrophils from the bone marrow.
- Some are growth factors (GFs) that promote the proliferation of cells such as neutrophils, macrophages, and fibroblasts. They bind to specific receptors on the surface of target cells. GFs may bind to different receptors on different cells and therefore mediate different effects, depending on the cells to which they bind.
- Cytokines that have been purified have been given the name “interleukin” followed by a number. If the amino acid sequence is unknown, the cytokine is named according to its biologic effect (eg, “tumor necrosis factor,” “macrophage inhibition factor”). However, since most cytokines have more than one biologic effect, descriptive names can be misleading.
- Cytokines are independent of the specific antigen that initiated the response.
Some of the cytokines that modulate the immune response are listed in Table 5-1.
In addition to those listed in Table 5-1, there are factors that are chemotactic for neutrophils, macrophages, and eosinophils and factors that evoke mitosis in uncommitted cells.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


