5
Caries
5.1 Introduction
Caries is a progressive, localised, destructive process in which:
- First, the mineral component of the dental hard tissues (enamel, exposed dentine and cementum) dissolves due to repeated disturbance of the equilibrium between the oral environment and the teeth, as a consequence of a localised decrease in pH because of microbial acid production in the presence of appropriate substrate (Section 5.3).
- Second, the organic component is destroyed. Unless the lesion becomes arrested, in time cavitation results.
If the 1–1.5 L of saliva produced daily (pH ∼7) was not supersaturated in calcium, phosphates, carbonate and OH−, the main constituent of the hard dental tissues, hydroxyapatite (Ca10[PO4]6OH2), would dissolve gradually.
The tooth crowns are covered with plaque, a slimy biofilm that harbours bacteria within numerous micro-environments.14 Some bacteria produce acids from the substrates present in the plaque, but the buffering action of saliva prevents a pH drop, until the acid production becomes excessive. At a pH of about 5.5 the environment is undersaturated with respect to hydroxyapatite, which then dissolves. Once the available substrate is used up, acid production stops, salivary action neutralises the pH and remineralisation starts. Repeated periods of demineralisation during the longer periods required for remineralisation result in a net loss of minerals from the enamel, leading to formation of the carious lesion. Initially, such a carious lesion consists of widened intercrystalline spaces,49 or “pores”, which manifest clinically as a chalky, opaque white spot. Cavitation starts after numerous prolonged acidic attacks.
Chemists describe caries in terms of the relationships between pH and mineral solubility. Microbiologists stress the interactions between bacteria and the food substrates. Psychologists and sociologists emphasize the role of the socio-economic status of patient/parent(s), dental anxiety,217 335 use of snacks and educational level,508 lifestyle,160 259 510 long-lasting conflicts in infancy, and low self-control.379
The essential description of the carious process in this chapter is, unless otherwise indicated, based on material drawn from several books and reviews.80 166 167 275 387 405 406 423 425 431 432 597 610 611 637
Genetics and Caries
Exogenous factors, such as fluoride, interfere with any genetic influences on caries. The frequent occurrence of caries obscures the contribution of genetics, which contributes to, among other factors, the morphology of the teeth.249 423 586
Studies on twins have revealed that the influence of genetics is smaller than that of the environment.542 Such twin studies often have weaknesses, such as small sample sizes and absence of surface-specific caries rates.95 Genetic screens of large populations have not been performed.542
The genetic make-up appeared decisive in animals:294 a difference in the immune response to specific bacteria is assumed,586 but the relationship between caries and immunity remains unclear.148 311 Caries susceptibility of rats seems to be genetically co-determined.503 Major host genes for susceptibility to caries may be located on chromosomes 1 and 2, and on 7 and 8 for caries resistance.429 Proline-rich saliva, an inherited trait, promotes the adherence to the teeth of early colonising streptococci (but not of Streptococcus mutans) and Actinomyces. Salivary immunoglobulins, another inherited trait, are bactericidal. Differences in dermatoglyphic patterns in persons with and without caries may also point to a heritable influence.36
5.2 Bacteria in Caries
5.2.1 Plaque Development
Soon after polishing a tooth, a biofilm of negatively charged salivary glycoproteins, the salivary pellicle, is seen to adhere to the tooth. Salivary bacteria (∼2.108/mL) are attracted towards this pellicle, and the sparse, reversible colonisation of the pellicle with a few species soon stabilises: microcolonies develop, which produce an inter-bacterial matrix that encloses other bacteria which do not have a capacity to adhere to the pellicle. The complexity of the plaque microbial population strongly depends on the salivary properties, crevicular fluid, mechanical factors, the substrate and other plaque-related factors (e.g. age of the plaque). It takes a few days for newly formed plaque to become cariogenic.
5.2.2 Composition of Plaque
Already at birth, the mouth is colonised by bacteria. The oral epithelium is in a state of continuous replacement; thus, bacteria that need to adhere to stable surfaces to multiply (e.g. S. mutans) only colonise the oral cavity permanently after tooth eruption.88 The then more complex plaque that forms contains bacteria associated with caries. Bacteria comprise 70% of plaque; the remainder 30% is composed of intercellular material derived chiefly from the bacteria, salivary proteins and epithelial cells, and also plaque fluid with calcium and phosphate, and, rarely, food remnants. The amount and rate of plaque formation varies between individuals due to differences in tooth brushing, sugar consumption, antimicrobial features of saliva, etc.
In experimental studies, germ-free animals fed on a cariogenic diet developed caries only after transfer of microflora from other animals. Long-term use of antibiotics or chemotherapeutic agents restricted the oral microflora and reduced the frequency of occurrence of carious lesions. Certain genera of oral bacteria can produce carious lesions in vitro. Huge numbers of S. mutans, Streptococcus sobrinus, some strains of Streptococcus mitis, Streptococcus sanguis, Streptococcus milleri, Lactobacillus and Actinomyces species have been isolated from in vivo carious lesions. Streptococci isolated from carious lesions were given the name Streptococcus mutans because of their oval shape, which seemed a mutant form.365
The plaque may contain several hundred bacterial species and the visible white layer (materia alba), which forms a diffusion barrier, particularly for substances with high-molecular weight. The barrier creates chemical gradients between the different layers of the plaque, which co-determine the kinds of bacterial species that may exist, and their activity, in the different plaque layers. There is a:
The plaque composition thus depends on the diffusion and reaction limits, on the type and supply of substrates and local oxygen tension. Strictly anaerobic areas exist next to aerobic ones. Water channels enable transport of bacterial products and nutrients between microenvironments within plaque.14 The plaque composition determines its pathogenicity and this is different for different types of tooth surface.
- Pits and fissures harbour many bacterial genera, but 80% are Gram-positive cocci, among which are large numbers of S. mutans, S. sanguis and Lactobacillus strains. Actinomyces species are also present. Candida albicans and other yeasts adhere to and dissolve in vitro hydroxyapatite to a greater extent than S. mutans,434 but usually few yeasts are present in the mouth, and mainly on the tongue; in vivo their cariogenic role seems small. After progression of the carious lesion into the dentine, S. mutans remains important (Table 5.1),449 but many other bacteria are also present.
- Approximal. Plaque accumulating underneath the contact points has a more varied and locally different composition.40 In particular it contains actinomycetes, followed by fewer numbers of Gram-negative bacteria and even fewer streptococci, but the numbers of S. mutans increase when caries develops. Because sound contact points also harbour all these microorganisms, plaque composition is a poor caries predictor.547
- Free smooth surfaces. Here, the mechanical action of the cheeks, tongue, mastication and tooth brushing restrict plaque growth. Only bacteria that are able to strongly adhere to the teeth initially become established. S. mutans, preceded by S. salivarius and Actinomyces, are important in smooth surface caries.
- Cervical. The most complex and thickest plaque is found cervically. The crevicular fluid and saliva maintain the plaque, which consist of more than 50 species, including Actinomyces and strict anaerobic bacteria in large quantities. Some of these play a role in root caries. In children, as the rate of crevicular fluid flow is low, the cervical plaque resembles the approximal plaque.
- Subgingival plaque differs from supragingival plaque and has a high percentage of anaerobes.
Table 5.1 Frequency (%) of occurrence of various bacteria in the proximala carious dentinal lesion449
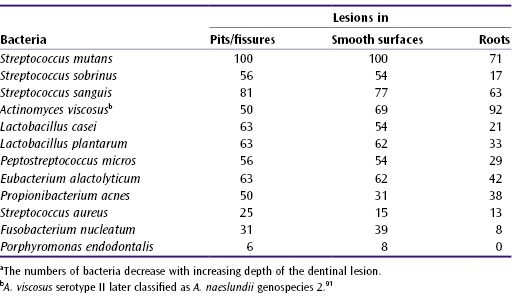
As stated above, the quality and quantity of the microflora (plaque) at a site that may be considered vulnerable to caries determines whether the disease develops or not.
5.2.3 Caries-Specific Bacteria?
In cross-sectional studies, plaque composition was moderately associated with caries. The majority of plaques are not cariogenic because they are insufficiently thick, contain few virulent bacteria, with little carbohydrate substrates, etc. Comparison of caries-free with caries-active subjects shows a strong association of caries with specific microbial species. Longitudinal studies of plaque compositions and caries show that caries progression in the enamel is strongly associated with persistently high numbers of S. mutans and, additionally, lactobacilli in dentinal lesions.
Until the 1960s, lactobacilli were considered to cause caries. However, it was shown that the consumption of varied diets, with or without cariogenic sweets between meals did not lead to any differences in the numbers of lactobacilli compared with a control group, which had less caries.432
Presently, the mutans streptococci in particular are thought to be the main culprits. Prior to the first sign of decay, the number of S. mutans in the area increases. The numbers of lactobacilli increase after caries initiation.144 390 547 Incremental caries progression, in particular on smooth surfaces, appeared associated with S. mutans,607 but was found to be greatest when S. sobrinus were present in greater numbers than S. mutans.248 Two-thirds of approximal surfaces did not show signs of caries when both S. mutans and S. sobrinus were present. This does not point to bacterial specificity of caries, but to the large production of acids by these bacteria.40 In “open” lesions, S. sobrinus prevails while in “closed” lesions with a less complex microflora, S. mutans predominates.138 Older plaques on healthy enamel also contain S. mutans, 318 which may not be present in plaques overlying newly developing lesions.
The numbers of S. mutans are only weakly associated with the risk of development of caries.564 In one study, children with high sugar consumption and caries rates had lower S. mutans counts than many of the caries-free children with low sugar consumption.105 Most of the mutans streptococci in plaques of caries-free children were, however, S. rattus instead of S. mutans strains.365 In one town, caries prevalence in children was associated with mutans streptococci and in another town with lactobacilli.204 Caries has been found to develop in the fissures of rat molars even in the absence of S. mutans and sugar.597 In Europe, Africa and North America, at least 70% of children less than 7 years of age harbour mutans streptococci: differences in the caries prevalence are due mainly to differences in the cariogenicity of the diet,647 and fluoride use.
The findings show that caries is not uniquely associated with one bacterial species. At least 300 species (possibly 1000) are indigenous to the mouth, but not many of them are dental pathogens. S. mutans and lactobacilli are significantly related to the onset and progression of caries and circumstantial evidence shows that they belong to the principal group of aetiological agents.365
In root caries, large numbers of anaerobic bacteria are present in addition to S. mutans, including some with proteolytic activity. Anaerobes assumedly play a role in caries progression. Weak positive correlations have been established with Actinomyces. The microflora consists of Actinomyces viscosus, S. mutans and S. sanguis, but S. sobrinus predominates, and in leathery root lesions, it is the lactobacilli.88 159 180 368 373
Experimentally, Actinomyces has been shown to produce root caries:158 high levels have been found to be associated (co-dominating with S. mutans) with greater numbers of root lesions.60 195 Actinomyces naeslundii forms 10% of the plaques on both healthy and carious roots,91 but has been, like other filamentous rods, ascribed an aetiological role. On caries-free roots, S. sanguis was found to predominate.195 The presence of both S. sobrinus and S. mutans was associated with more root caries than S. mutans alone.368 Aamdal-Scheie et al. found that plaques on healthy and carious roots were identical2 and had the same levels of S. mutans and lactobacilli.195
When carious dentine is lost, the root becomes recolonised.530 In case of “arrested caries”, the amount of plaque decreases.529
These findings do not point to a bacterium-specific aetiological basis for root caries.650
5.2.4 Transmission of Cariogenic Flora
Oral infection in children is related to frequent contact with large numbers of bacteria from the mother (“cuddle effect”) and other caregivers108 135 319 within the discrete period between 18/19 and 31 months (the “window of infectivity”).108 596 But the presence of caries testifies that children aged 9–10 months are also already infected630 and S. mutans was detected in 25% of one sample of pre-dentate children.413 Caries prevalence rates in children follow those in mothers more than those in fathers.89 Maternal caries best predicts caries in 11–12-year-olds.621
A study of mother–child dyads during the first 7 years of life revealed that two-thirds of the children had acquired S. mutans and one-quarter had acquired S. mutans plus S. sobrinus, while all the mothers possessed both species.356 The correlation between numbers of mutans streptococci in maternal and children’s saliva is low:498 70% of 3-year-old children harboured S. mutans, compared with 40% when mothers’ microflora was suppressed for a period spanning a few years. If 15-month-olds harboured S. mutans, the probability of caries at the age of 3 years was 75%.319 In one study, children free of mutans streptococci until age 5 had more sound teeth at age 11 than children who acquired the bacteria earlier.581
When the number of S. mutans in a mother is reduced permanently, her child’s mouth is colonised at a later stage or not at all. Bacteria newly introduced in the mouth are more likely to get established in children than in adults with their highly colonisation-resistant, optimally adapted flora. In adults, a new bacterium rarely spreads from the inoculation site and becomes undetectable within a few weeks. In contrast, tooth eruption creates conditions favourable for colonisation. In 19–31-month-old children a new bacterial colony is established relatively easily.
5.2.5 Virulence Factors
The amount of acids, the rate of production and the time for which they remain on the tooth surface co-determine the cariogenicity of plaque. In the presence of sugar, plaques with larger numbers of mutans streptococci quickly produce much acid. Virulence factors of such plaques are as follows.
- Intracellular polysaccharides formed from sugars within S. mutans (in S. sobrinus these sugars are largely absent)597 are fermented after exhaustion of the external sugar supply, thereby prolonging periods of low plaque pH.
- Extracellular polysaccharides make the plaque stick to the smooth tooth surfaces. Mutan, produced exclusively by S. mutans from saccharose, and glucan produced by S. sobrinus, are important components of this system. Glycosyltransferases from the saliva, new pellicle and S. mutans catalyse the synthesis of glucan linkages from sugars. Interactions between glucan linkages leads to the formation of extracellular polysaccharides, which adhere to the teeth and then incorporate other bacteria.500
- Sucrose-independent surface protein antigens I/II (PAc) participate in the initial adherence of S. mutans:212 these bacterial proteins interact with lectins in the pellicle.560
- S. sobrinus excretes a virulence-associated immunomodulatory protein (VIP), which inhibits the host’s response.140
- High catabolic activity, manifest at a low pH, of notably S. mutans and S. sobrinus, ensures a high concentration of acids.
- Autoselection. Acid-producing bacteria are aciduric and multiply at a low pH, which adds to the process of bacterial selection that alters the plaque composition.
The cariogenic bacteria absorb and ferment sugars, form acids, secrete H+ through their cell walls and take up K+, thus maintaining their electrochemical equilibrium and preventing acidification of the bacterial cytoplasm. At lower pH, some of the plaque acid does not dissociate and this acid is taken up in the bacteria. Within the somewhat less acidic bacteria, the acids dissociate and H+ is released. Ultimately the capacity to release H+ fails, the internal pH drops and the metabolism of the bacterium stagnates, which prevents the pH in the plaque from become lower than about 4.107 S. sobrinus produces more acids at a low pH than S. mutans.136
Acid formation and secretion of H+ by S. mutans is governed under anaerobic circumstances by fluoride, which behaves as a non-dissociated acid: the degree to which this occurs is determined by whether the bacterial species thrive in an acidic or neutral pH environment.195
Because of bacterial resistance to acids, erosion (Chapter 6) may alter the plaque composition. Some research indicates in such circumstances S. mutans and S. sobrinus are present in proportionately high numbers, but this has not, however, been confirmed.251
None of the virulence factors are unique properties of S. mutans, with the exception of mutan production, which supports the non-bacterial species-specific aetiology of caries. However, if S. mutans are present in substantial numbers, which depends on the diet, carious lesions may develop relatively rapidly. The group of related oral bacteria known as mutans streptococci, which include S. rattus, Streptococcus cricetus, and S. sobrinus, is implicated as the primary aetiological agent of caries, and within this group S. mutans and S. sobrinus are the species most commonly isolated from carious lesions.137 365
5.3 The Substrate
Many plaque bacteria survive on a diet of salivary glycoproteins, but the rate of plaque formation in humans on parenteral nutrition increases quite rapidly on resumption of oral feeding. The plaque swiftly assimilates low-molecular compounds (sugars, peptides) and a small proportion of proteins and polysaccharides after enzymatic breakdown. Substances other than carbohydrates, fluoride, calcium and phosphate seem to be of minor importance as far as direct uptake by plaque is concerned.
Sugars may be consumed a number of times daily, and then in high concentrations. Consequently, the plaque synthesises polysaccharides and produces acids, which promote plaque growth, auto-selection of mutans streptococci, and caries. Frequent sugar consumption results time and time again in acid production and shortens the remineralisation periods: under these circumstances carious lesions then develop or progress.
5.3.1 Sugars
Polysaccharides, such as starches, usually remain too briefly in the mouth to be sufficiently transformed into sugars that can diffuse into the plaque. The buffering capacity of the saliva and plaque fluid easily neutralise the acids thus formed. In contrast, free dietary sugars immediately penetrate the plaque and are absorbed by the bacteria and hydrolysed. These mono- and disaccharides are: saccharose (=sucrose = “sugar”), glucose, fructose, maltose, lactose and galactose. Pollard showed that the plaque pH fall was largest after sugar consumption, followed respectively by pasta, ripe banana, white bread, cornflakes and the sweetener sorbitol.469 The pH drop is smaller and shorter lasting in children than in adults.589
5.3.2 Saccharose
Sucrose provides 15–20% of the dietary caloric value, other saccharides a few per cent. Caries development therefore depends mainly on the consumption of sugar, although glucose, fructose and lactose are almost as cariogenic. Sucrose, a disaccharide of fructose linked to glucose, is refined from the juice of sugar cane and beet. Hydrolysis of the molecule frees the energy from the fructose–glucose bond, which the bacteria use to synthesise the extracellular polysaccharides, mediated by the glycosyltransferases which direct the glucose to the growing polysaccharide.
Sucrose is used rapidly and effectively:
- For synthesis of extracellular polysaccharides. Without sugar, adherence of plaque to the tooth is reversible; with sugar the bond becomes insoluble in water.
- As an intracellular substrate, after splitting and uptake, which serves as an energy source when the external food supply is exhausted.
- For intracellular synthesis of cell membrane material, etc.
The hetero-fermentative bacteria in “resting plaques”(i.e. in times where there is nothing else other than saliva and crevicular fluid in the mouth) form acetic, lactic, propionic, butyric and formic acid, alcohol, carbon dioxide, and other products. In the presence of rapidly fermentable sugars, the concentration of lactic acid increases quickly, more than that of other acids.
In one study neither the amount nor the frequency of sugar consumption differed between children with and without caries,341 which stresses its multifactorial character; but generally the main distinction between individuals with and without caries is more frequent consumption of cariogenic food, that is sucrose, between meals.267
5.4 The Initial Lesion (Enamel)
5.4.1 The Carious Process167
With respect to the enamel at a pH of 7, saliva is supersaturated with calcium and phosphate. Enamel closely resembles hydroxyapatite, but contains a variety of impurities.
Some impurities (fluoride, zinc) are mainly superficially located; others (sodium, carbonate) are far from the enamel surface or are distributed evenly (strontium, vanadium). Many impurities are soluble, but when they form part of the crystal lattice they are not easily dissolved, for example fluoride which has substituted for the hydroxyl group during odontogenesis.431 Post-eruptive administration of fluoride may lead to formation of calcium fluoride, or fluorhydroxyapatite, which is somewhat less soluble than hydroxyapatite.340 Aluminium makes the enamel less soluble in acid,313 but a fluoride toothpaste was found to be more effective than an aluminium paste.234 Copper, strontium and barium reduce the risk125 126 127 145 502 668 701 and magnesium, zinc and selenium increase the risk of developing caries as well as progression of developed lesions.126 442 The knowledge of the action of trace elements such as lead, iron, and molybdenum in the teeth is incomplete.
Bacterial acids at the plaque–enamel junction make the environment of the tooth undersaturated at the “critical” pH of 5.5 (a pH below 6 is potentially harmful).613 After a sugar solution rinse, the pH underneath plaque drops to about 4.0, and remains low for 0.5–1 hour,348 or even longer at sites protected from salivary flow.280 The pH depends on the buffering capacity of saliva, which is determined mainly by the bicarbonate (HCO3−) system:
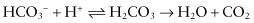
The HCO3− concentration increases considerably on salivary gland stimulation. The buffering capacity of the phosphate system is insufficient because of its low concentration.275
Under acidic plaque, the enamel demineralises when the buffers are depleted and H+ ions from the acids penetrate the interprismatic substance. First the intercrystalline spaces enlarge due to partial dissolution of the individual crystal periphery. Later the thin overlapping portions of the perikimata dissolve with further enlargement of the intercrystalline spaces. The process advances along the prisms into the deeper layers of the enamel. The mineral dissolution creates pores in the enamel, which act as transport channels through which dissolved minerals diffuse to the outside of the enamel and are carried away with the flow of fluid from the pulp.28
Dissolved minerals from the surface are replenished with minerals from the subsurface layer, which becomes poorer in minerals.405 The superficial (30–50 µm) enamel therefore remains largely intact and provides surface protection, also due to the formation of macromolecules under the influence of proline-rich saliva and other salivary inhibitors, which cannot penetrate the deeper parts of the enamel, although up to 5% of it consists of pores; but underneath, mineral loss is greater. Explanations have also been offered for why the surface is, at large, unaffected.98 The surface is thought to be protected by absorbed proteins, which would contain more minerals than the deeper layers and contain more fluoride and less carbonate, making it less soluble.349 Or a gradient may exist in solubility rate, and low fluoride concentrations considerably affect the solubility gradient.606
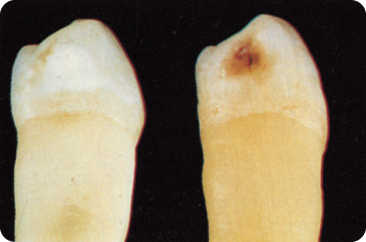
The mineral loss described above makes the lesion visible as a “white spot” after blowing air on the tooth surface in the earliest phase of caries development; this is because fluid within the pores maintains the translucency of the enamel. Later, the white spot is clearly visible without air-drying the tooth (Figure 5.1), signifying an enlarged subsurface pore volume. Microcavities may develop.167 The pores encourage progression of the process, chiefly along the enamel prisms and the lines of Retzius.606
Figure 5.1 White spot caries (initial lesion) and brown spot (discoloured white spot).
Fluoride-containing apatite dissolves at a pH lower than 5.5, which implies that the enamel is less frequently exposed to an harmful pH (the pH must be lower than 5.5 to be critical) and because the pH returns slowly to neutral, the critical period is shorter. The margins of the white spot and plaque correspond. In the interdental contact areas, the white spot is oval and resembles cervically a half moon. The enamel is most porous at the centre of the subsurface lesion, following the direction of the enamel rods from the surface to the deepest point.
When the external sugar supply and the intracellular polysaccharides are exhausted, the salivary buffering mechanisms slowly raise the plaque pH to neutral, aided by the rinsing of the tooth surface by saliva and dilution. Pellicle proteins promote the remineralisation by attracting salivary calcium ions.645 The local environment becomes saturated and minerals precipitate superficially into the pores. Demineralisation is retarded in concert with the concentration of calcium and phosphate ions in saliva and plaque fluid. A sucrose rinse increases the plaque calcium concentration by a factor of 2.5 and also increases the phosphate concentration. Ions released from the enamel slow down the demineralisation by saturating the plaque fluid with ions.595 645 The fluoride concentration in plaque remained unaltered, indicating supersaturation.595
Half an hour of demineralisation requires several hours for complete remineralisation. Under laboratory conditions, equilibrium was found to be established when the pH was lowered no more than six times for 30 minutes each per day.600 Passive transport of salivary and plaque Ca2+ and H2PO4− ions down the concentration gradients into the lesion is the main driving force for remineralisation, but solubility constraints limit the mineral ion concentration in the remineralising fluid.113 Moreover, a mineral shortage exists, because during the acid attacks ions from the enamel are lost to the oral cavity.275
In vitro, five factors limit the remineralisation within the depth of the lesion:339
During remineralisation, pigments from dietary components may become incorporated into the lesion. The white spot then becomes a brown spot (Figure 5.1). The amount of mineral that can dissolve depends on the saturation of the solution, and is governed by the thermodynamic ion activity product of a given solution.113 The extent to which fluorapatite is dissolved, in a given solution, is less than that of apatite. If the hydroxyl ions in the enamel were systematically replaced by fluoride ions during its formation, a small percentage of the enamel would consist of more tightly formed fluorapatite.431 The local effect of fluoride by far exceeds this systemic effect.691 Fluoride in the plaque and saliva enhances the precipitation of CaF2 and some fluorapatite is formed. Precipitation of minerals including pH-resistant CaF2 plugs the entrance to pores, which inhibits the remineralisation of deeper parts.600 With dissolution, the CaF2 provides a slow-release fluoride depot.501
Enzymatic612 destruction of the organic component starts after mineral dissolution. Collagen, which forms a small proportion of enamel (10 times less than dentine), must be free of minerals before it can be destroyed. The enamel lamellae are organic (hypomineralised) linear defects that cross the enamel. Contrary to the notion that these lamellae are pathways along which caries can easily progress,460 they are quite resistant to acids.71 The exact role of proteases in enamel caries is not known.82 115 652 Enamel cracks (Chapter 9) may visually resemble the lamellae, but they allow ingress of cariogenic bacteria.670
5.4.2 Histology and Chemistry of the Initial Enamel Lesion167 432 549 551
Initially, the enamel pores are about 20–50 µm deep. Fresh acid attacks enlarge the pore volume: the crystallites become smaller and hollowed out lengthwise, the enamel rods dissolve peripherally, and the pores become deeper. “Focal holes” (micropits) and small areas with irregular destruction develop.216
In the presence of acids, the hydroxyapatite (Ca10(PO4)6(OH)2) dissolves into Ca2+ and H2PO4− and H2O. Other reactions also occur. Brushite (CaHPO4.2H2O) is formed, which in the presence of fluoride is rapidly converted into fluorapatite. Phosphate as a tooth mineral exists in the basic form of PO4− but under mildly acidic conditions, dissolved phosphate ions are predominantly in a more acidic form.113
An acid concentration gradient exists in the enamel from the outside to the inside. The deeper, less acidic but weak bacterial acids dissociate at a relatively high pH and may therefore be active in the deeper layers. Dissolved calcium and phosphate ions diffuse to the outside, where they precipitate as acidic calcium phosphate, thereby maintaining the surface integrity.275
Smooth Surfaces
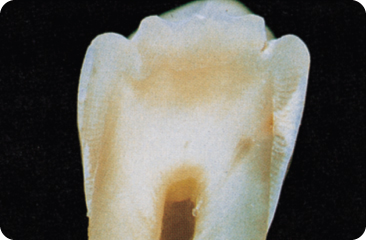
The initial lesion is conical, with its base towards the surface (Figure 5.2). Demineralisation is worst in the centre, the oldest part of the lesion, and becomes gradually less deeper within the lesion, away the periphery. The deeper the central pores, the wider is the lesion peripherally.80
Figure 5.2 Initial carious lesion in a smooth tooth surface; the demineralisation defect (white) at the right side is conical in shape, with its base towards the surface of the tooth. The dentine is just involved, visible as a small, brown discoloured area.
Fissures
White spots develop back to back in both walls of the fissure (Figure 5.3). The shape of the occlusal fissures varies and the direction of the enamel rods is not always perpendicular towards the dentino-enamel junction; but the shape of the fissural carious lesion is principally conical. After some time, the two demineralised areas merge underneath the fissure. In small, artificially created grooves, less mineral was lost from the walls than in broader grooves, which might have been because the diffusion of acids to the inside and that of the mineral ions to the outside was restricted.332
Figure 5.3 Initial carious lesion in a fissure; the defect is present on both walls of the fissure. Again, the dentine is involved.
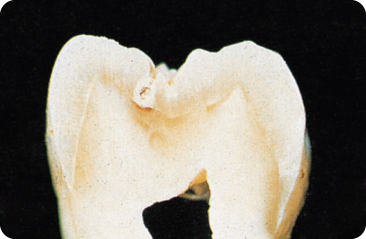
Bacteria are absent in the initial enamel lesion: the result of a demineralising acid front.92 Microscopically, four zones are distinguishable:
The deepest enamel layer may be normal.
5.5 Progression of the Carious Lesion167
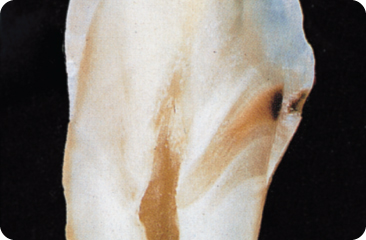
- Enamel. Demineralisation advances little by little towards the dentine (Figure 5.4). Cavitation, in general, does not start before the dentine is involved,7 and is not seen when, on radiographs, the demineralisation front extends to and even beyond the dentino-enamel junction. In a sample of caries-active patients, the approximal surfaces with demineralised areas on radiographs showed discontinuity of the enamel.367
Figure 5.4 Smooth surface caries lesion showing progression in all directions. The defect in the enamel is conical in shape with its base towards the surface. The width of the lesion in the dentine is the same as that of the lesion at the enamel surface. The two light brown areas in the dentine represent sclerotic tissue.
Many cervical white spots remain unchanged in the long term.610 Backer Dirks found that within 7 years, 10% of initial lesions on smooth surfaces of children cavitated, 40% remained unchanged, and 50% became indistinguishable from the surrounding enamel. Considerably more fissure lesions cavitated and the process was faster.42 A minority of approximal lesions reaching the dentine, and brown discoloured fissures, healed within 1–5 years. The rate of progress of the approximal lesions was slow, particularly when confined to the outer enamel.461 In another study, within 3 years, 15% of approximal enamel lesions had healed after application of preventive measures, 35% had stabilised, and 50% had progressed.266
- Dentino-enamel junction. Before the lesion reaches the junction, dentinal changes occur. Deposition of minerals causes narrowing of the tubules, creating a hypermineralised zone. At this stage, the pulp becomes involved via communications with the tubular fluid and the odontoblastic processes (Figures 5.5 and 5.6). Thereafter, bacterial products penetrate through the enamel and reach the dentine. The critical pH for the dentine is 6.0–6.5 and freshly exposed dentine does not contain much fluoride.158
- Dentine. The connections (anastomoses) between the tubules have been considered to be pathways for lateral expansion of the demineralisation process along the dentino-enamel junction. However, in the precavitated stage, the width of dentine lesion does not exceed that of the enamel surface lesion,76 and demineralisation does not spread along the dentino-enamel junction,48 but follows the tubules.80
Figure 5.5 The carious lesion has reached the pulp.
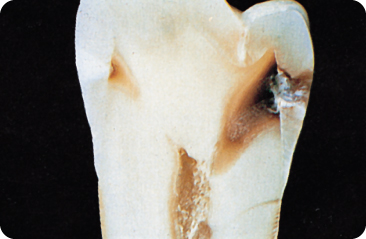
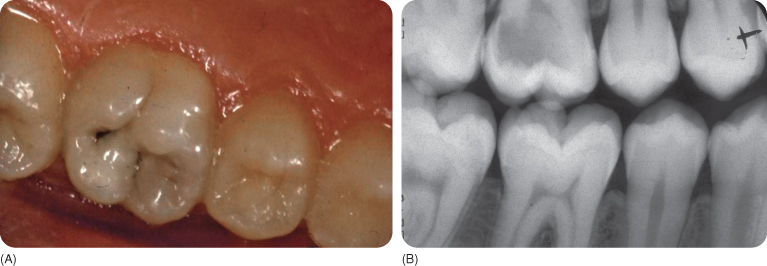
Figure 5.6 (A) The occlusal surface shows a discoloured distal fissure, but seems otherwise more or less intact. (B) An X-ray of the same tooth showing a large carious lesion (“hidden caries”) in the dentine underneath the small occlusal entrance of the lesion.
The process, at first a demineralisation front, progresses in the direction of the pulp. The hypermineralised zone protects against lesion progression, but the tubular blockade fails if the (repeated) attack is strong enough. The base of the conical, truncated lesion is at the dentino-enamel junction. An enamel cavity is commonly still absent. At this stage the odontoblasts produce tertiary dentine (Section 5.5.3).
When the process extends beyond the dentino-enamel junction and an enamel cavity has developed, the dentinal lesion spreads along the junction, undermining the enamel. At this stage the deeper dentinal layers are already demineralised. Lateral spread is also observed in microcavitated lesions, which are invaded by bacteria. It is associated with softening of the dentine, which depends initially on the way in which the bacterial acids penetrate the enamel prisms.155 By this stage demineralisation has reached the middle third of the dentine.
Once there is frank cavitation, the inner half of the dentine is demineralised and the outer half infected.155 Proteolytic enzymes degrade the collagen matrix that is denuded by demineralisation.
Once exposed by the acids, the organic matrix is destroyed by non-specific proteases after breakdown of the electrostatic bonds of the side chains.314 315 Demineralised dentine shows considerable loss of collagen, unrelated to specific bacterial species. Great numbers of Actinomyces have been found to be present in dentine with largely exposed organic component.650
The non-collagenous organic dentine components may counteract remineralisation: their removal with sodium hypochlorite in vitro was found to promote remineralisation.265
Among the proteolytic enzymes are matrix metalloproteinases that are capable of degrading native and denatured collagen type I and might be activated by bacterial acids. Metalloproteinases are also present in plaque, saliva and crevicular fluid. Metalloproteinase activity is seen in extracts from demineralised dentine, but appeared unrelated to the collagen loss. Activity of a cystein proteinase (cathepsin B) which degrades collagen in mildly acidic conditions, correlated positively with activity of metalloproteinases.651
5.5.1 Histology of Dentine Caries
The dentinal lesion comprises six zones:
Bacteria penetrate the dentine only after cavitation. Layers 3, 4 and 5 are absent where the carious process has penetrated very deep into the tooth.551
5.5.2 Macroscopic Appearance
In enamel, white and brown spots and cavitation are the macroscopic signs of caries. Carious dentine is more or less discoloured. Before restoration, the layers of destroyed and infected dentine are removed (Section 5.12.2). The remaining demineralised dentine layer must be hard enough to support the restoration.185
5.5.3 Pulpal Reactions
The relationship between clinical caries and the histopathological condition of the pulp has not been “classified” for a variety of reasons,80 559 including: marked variations in pulpal reactions,337 lack of information on caries activity, and artificial loss of enamel prior to pulp examination. The organic and inorganic phases are difficult to study simultaneously.
Yet, some conclusions seem warranted.80 The pulpal reaction starts when white spots develop. The odontoblasts are smaller in size underneath any active caries lesion extending to the dentino-enamel junction and cellular proliferation is seen in the cell-free zone of the pulp. These changes have not been seen underneath arrested caries lesions,80 implying that they are reversible.
The pulp–dentine complex shows different responses that interact in a complex way. Their relative contributions are critical for determining the fate of the pulp–dentine complex.559
- Injury responses. The dentinal matrix and solubilised minerals buffer the hydrogen ions, but the odontoblasts underneath active enamel lesions show cytoplasmic changes and decreased activity, and dentinogenesis may cease.
- Defence reactions. A defensive inflammatory pulpal reaction is followed by reactions that determine the degree of healing and repair; tertiary dentine modifies the permeability of the dentine. As bacterial ingress progresses, the inflammatory pulpal reaction becomes more pronounced and acute and can compromise pulp survival.
- Repair reactions. Odontoblasts secrete focally reactionary dentine in milder (active)77 carious attacks. When the odontoblasts do not survive, a new generation may arise from differentiation of other pulpal cells and form reparative dentine.
Bacterial acids and other metabolic products are, as irritants, directly responsible for tertiary dentine formation, hence named irritation dentine. Growth factors (which have a limited diffusion distance) and cytokines present in the dentine matrix stimulate the odontoblasts and presumably the progenitors of second-generation odontoblasts.
Tertiary dentine is already formed in non-cavitated caries, somewhat apical of the enamel lesion in relation to the direction of the tubules.551 In some cases irritation dentine is absent there, but large amounts of it are formed in the vicinity.337 The more active the carious process, the more irregular is the reactive dentine. Under slowly progressing caries, tertiary dentine resembles secondary dentine. When lesions progress rapidly, the odontoblasts are damaged and no tertiary dentine is formed.80
Inflammatory and immunological pulpal reactions follow the ingress of microbial antigens, toxins, allergenic bacterial agents and bacteria through the tubules. When pulpitis develops, it may be symptomless.80 337 559 In one sample of deciduous teeth with exposed pulps, one-third showed transitional pulpitis, but two-thirds of deep carious teeth without pulp exposure had a normal pulp or transitional pulpitis.151 The pulp becomes significantly diseased when caries invades the tertiary dentine.479
The degree of both reversible or irreversible pulpal inflammation cannot be assessed non-invasively,80 necessitating complete removal of affected dentine. The status of the pulp is no indication of the severity of the caries process, unless the pulp is severely infected or necrotic.
5.6 Root Caries
Gingival recession exposes the cementum to the oral environment: root caries may then develop (Figure 5.7). Dentine has a lower mineral content and crystallinity than enamel and is therefore more soluble. Bacterial invasion occurs along the direction of the Sharpey’s fibres, which are degraded, and causes a rather wide subsurface than deep lesion,256 528 in relation to dentine sclerosis in older persons.
Figure 5.7 Root caries (plus approximal caries, left panel) and approximal caries (right panel).
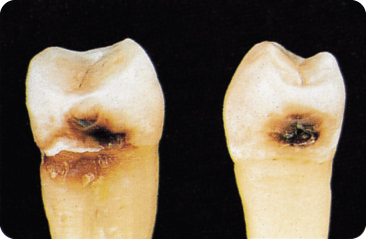
Multiple small demineralisation foci528 and mini-channels or clefts reaching into the cemento-dentine junction are characteristic. Hypomineralised areas allow an exchange of fluid between the dentine and oral cavity. Underneath, a thin hypermineralised surface layer contains areas with destroyed collagen. The lesion spreads and undermines the cementum.
A brown-coloured lesion signifies a slowly progressing lesion whereas a light colour indicates an active lesion.373 Coronal and root caries may be considered different entities because the latter is present: (1) sometimes almost exclusively on roots of teeth free of coronal caries; and (2) to the same degree in persons with and without fluorosis.201 Root caries correlates moderately with coronal caries (r = 0.60).180
Root caries accounted for some half of the carious lesions in the Middle Ages, including in the deciduous molars.299 448 653 663 There is a strong relationship with periodontal disease, as found in tribes of New Guinea, where coronal caries is uncommon.516
Gingival recession is a precondition, but it does not explain the prevalence of root caries.214 Medical, socio-economic and behavioural variables partly explain lesion development.58 Alterations in the oral condition also play a role. The salivary buffering capacity is reduced when the salivary flow decreases (see Section 5.7.3). Bacterial production of acids and enzymatic proteolytic activity of the anaerobic bacteria cause root caries.2 147 528 Host-derived salivary proteases are also involved. The collagen fibrils must be almost free of mineral before they are degraded but for remineralisation sufficient mineral must be present on the fibrils.314 315
Ongoing eruption of the teeth and recession of the gingiva shift the area covered with plaque apically: thus alternating sound and carious zones may exist.
5.7 Some Risk Factors
Several groups are especially vulnerable to caries, including people with mental and physical disabilities and anxious patients. Several other factors may exert a large influence on the development of caries, and these are described in the following subsections.
Anxiety lowers the salivary flow rate or alters the salivary composition,507 which decreases its rinsing and buffering actions, and concentrations of anti-bacterial enzymes.432 Emotional stress reduces the serotonin level in the brain, which is restored by consumption of cariogenic carbohydrates. Children with caries have higher urinary levels of catecholamines, a measure of emotional stress.636 Whether anxious people develop more caries than others is questionable.59
5.7.1 Chronic Malnutrition
A shortage of proteins and fats during growth may permanently reduce the amount of stimulated salivary flow and buffering capacity, impair calcium and protein levels in stimulated and decrease immunological and agglutination defence factors in unstimulated saliva.282 283 Malnutrition may result in caries if xerostomia (Section 5.7.3) is a consequence.282 Anomalies of enamel structure as a result of malnutrition, such as in coeliac disease,473 are associated with increased risk of caries.353 413
5.7.2 Inborn Errors of Metabolism and Other Diseases
Some rare disorders of amino acid metabolism require consumption of cariogenic diets for normal growth or can lead to chronic xerostomia.
Phenylketonuria (PKU)
PKU is uncommon (1 : 10 000). The amino acid phenylalanine needs a liver enzyme (phenylalanine hydroxylase) to convert it into tyrosine. When this enzyme is lacking, phenylalanine accumulates in the body fluids, with consequences such as progressive mental retardation and epilepsy.
PKU patients are treated with a diet poor in proteins and rich in carbohydrates, and with a mixture (drink) of amino acids, free of phenylalanine. The acid and therefore sweetened drinks are consumed at least twice a day. To guarantee full energy intake, snacks and sweetened beverages must be consumed, often every 2 hours.118 The artificial sweetener aspartame (a dipeptide of phenylalanine) is contraindicated.420 Phenylalanine in the diet reduces likelihood of plaque formation but the risk of caries development might be increased: the literature is inconclusive.118 However, children with PKU receiving fluoride had markedly less caries, an effect absent in unaffected siblings.671
Enamel hypoplasia is associated with PKU, but dietary improvements may have reduced the risk of development of enamel defects.118
Other Inborn Errors of Protein Metabolism
Several very rare disorders are due to lack of various enzymes. Abnormal accumulations of the (intermediates of) amino acids necessitate a low protein and a high carbohydrate intake.118
These disorders include maple syrup urine disease (urine has a caramel smell), organic acidaemias (patients may require dialysis), urea cycle disorders (ammonia accumulates because no waste nitrogen is formed), homocystinuria (homocystine and methionine accumulate), and tyrosinaemia.118
Glycogen storing disorders (four types of protein and/or carbohydrate disorders) cause excessive storage of glycogen in liver/muscles and hypoglycaemia. Extra glucose is needed. There are few anecdotal reports of high caries rates in affected children. Administration of cornstarch supplement results in sticky debris on the teeth.118
Diabetes
In diabetic people in whom hyperglycaemia is prevented295 and dietary precautions are taken, the caries prevalence is similar to the population average. However, samples of people with diabetes were found to have more restored tooth surfaces than controls.10 397 Poorly controlled patients may develop more caries, associated with the increased presence of oral fungal microorganisms.295 Hyperglycaemia strengthens the association between caries and mutans streptococci and lactobacilli.588 In non-insulin-dependent diabetes, there is no effect on the caries and yeast prevalence, despite the protective effect of saliva being partly lost.119 In older people with diabetes, a tendency for more active caries may exist, but an increased prevalence has not been reported.359
Diabetic rats develop root caries despite a diet poor in carbohydrates. The crevicular fluid might contain much glucose.483 Compared with controls, people with diabetes were found to have both more and fewer cavities.609 Unless diagnosed late, people with diabetes do not have more (root) caries than other people, owing to the insulin therapy and consumption of a (probably) non-cariogenic diet,188 203 397 422 perhaps with the exception of lingual caries.468 The number of cariogenic bacteria is “normal”.609 Saliva parameters may be identical to those in people without diabetes and those with well-controlled diabetes.587 However, in people with diabetes, the saliva may contain more fructose609 and, at the start of the disease, more glucose and IgG.61
People with diabetes may also have a reduced stimulated salivary flow due to use of antihypertensive medication.609 698
Fructose Intolerance
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


