3
Developmental Structural Anomalies of Enamel and Dentine
3.1 Introduction
A variety of causes are responsible for the developmental structural anomalies of enamel and dentine. For better insight into these anomalies, the development and structure of the teeth are first briefly reviewed here.
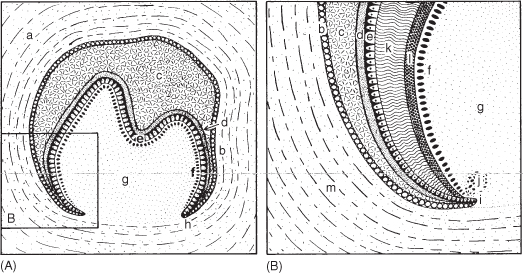
Odontogenesis starts with development of outgrowths from the oral epithelial band that lines the (future) jaws, from which the tooth buds develop. The tooth bud is initially cap shaped, which then grows to form the bell-shaped enamel organ. The enamel organ surrounds the mesenchymal dental papilla and has four layers: the outer epithelium, stellate reticulum, stratum intermedium and inner epithelium.
Enamel
After predentine formation by specialised cells of the papilla (the odontoblasts), cells from the inner enamel epithelium transform into ameloblasts (Figure 3.1). These ruffle-bordered cells: (1) produce the organic enamel matrix, (2) resorb the greater part of the matrix and (3) mineralise (through mineral deposition) the remaining matrix. The enamel production proceeds in an incisal/occlusal to cervical direction. Next, the ameloblasts disappear and the as-yet partly mineralised enamel825 undergoes “maturation”, a process that starts before and continues after eruption.
The enamel matrix consists mainly of two proteins: the amelogenins and, to a lesser degree, the enamelins. The amelogenins regulate the formation of the hydroxyapatite crystallites. During the calcification, the amelogenins are split step-wise by proteases secreted by the ameloblasts,517 and new proteins are added until early maturation, when few new proteins are formed.825 Two groups of enamel proteases exist: a matrix metalloprotease called enamelysin and a serine proteinase. The amelogenins are cleaved by enamelysin, from the inner to the outside side of the crown, and disappear totally during the maturation of the enamel. The enamel matrix contains several amelogenins, as a result of their destruction and differences in transcription.517 The mineralised enamel undergoes final calcification as the remaining proteins are removed,825 but the acidic enamelin concentration increases.218 The loss of matrix protein creates pores, which are subsequently filled with mineral. The enamel crystals slowly expand, filling the spaces formerly occupied by the proteins, and the local pH changes from mildly acidic to near physiological.823 The longer the developing enamel remains partially mineralised, the greater the likelihood that the tissue will be damaged, which might explain why the incisal edge shows anomalies more often than the cervical region.219 There is a time-dependent difference in enamel maturation in males and females.1
The enamel is composed of almost parallel, about 1 mm long rods (enamel prisms) with diameter of about 4 µm, composed of small hydroxyapatite crystals. In a cross-sectional view, the rods resemble keyholes. Their direction is perpendicular on the outer surface of the enamel, and occasionally at an angle of 30 degrees and in the cervical region it is almost greater than 45 degrees.121 The minimal spaces between the prisms are filled with interprismatic material, which consists of very small hydroxyapatite crystals, water and organic components.
The enamel surface of newly erupted teeth shows numerous fine horizontal ridges (the perikymata) separated by very small grooves (“imbrication grooves of Pickerill”),738 which correspond to the growth lines within the enamel. The growth line that marks birth is named after Retzius. A lower premolar shows some 30 perikymata/mm at the cervical site, gradually declining to 6–7/mm near the occlusal surface.848
Figure 3.1 (A) Schematic of the bell stage of development of a tooth. (B) Magnified view of the inset in (A).
Dentine
The odontoblasts first produce fibroblasts and extracellular collagen fibres. Collagens consist of microfibrils of some 20 protein families. Type I collagen constitutes 85–90% of the mass of the dentinal framework.209 Collagen type V is associated with type I collagen molecules that have formed into fibrils209 and is present in small amounts.6 Type III collagen is found in the predentine.209 In cross-sections, the bundles of collagen fibres (the Korff fibrils) become orientated perpendicularly on the basal membrane (the basal lamina situated between the mesenchyme and inner enamel epithelium) that separates the papilla from the inner enamel epithelium. The Korff fibrils constitute the matrix for the first-formed dentine, the mantle dentine (this is 150 µm wide, lacks phosphoryn, and is less mineralized than the later formed dentine). Mineralisation of the matrix, which until then is called predentine, occurs in the form of round deposits of calcium salts after the basal lamina is removed. Dentine formation thereafter takes place in the absence of Korff fibrils. Dentine contains a much higher proportion of organic substances than enamel. Many uncertainties still exist in regard to the inherited anomalies of dentine.107
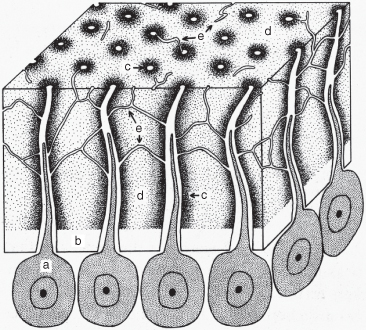
A very large number (up to some 18 000/mm2 and locally even thousands more) of dentinal tubules run from the pulp to the enamel and cementum (Figure 3.2) and are filled with fluid and, within about the proximal half, extensions of the odontoblasts. In the axial parts of the dentine, about 10% of the extensions of the odontoblasts within the tubules are accompanied by nerve endings. The tubules are surrounded by peritubular dentine, which is more calcified than the intertubular dentine, and are interlinked by transverse connections. Imperfect calcification of the dentine, in particular near the enamel, results in many regions with abundant organic material, called interglobular dentine.
Figure 3.2 Schematic of the dentine.
The Tooth Root and Cementum
Cell division above the “cervical loop”, which consists of only the inner and outer epithelium of the enamel organ (Figure 3.1B), moves the cervical loop apically, leaving a shaft, the “sheath of Hertwig” of inner and outer epithelium surrounding the mesenchymal papilla. On the inside of Hertwig’s sheath, the outer cells of the dental papilla transform into odontoblasts, which produce the root dentine. Next, the sheath becomes a loosely connected network, which in cross-sections is visible as the “islands of Malassez”. The mineralised dentine induces migration of the nearby mesenchymal cells of the follicle (a loose connective sac) through the network, which become cementoblasts. These cells produce a collagen cement matrix, which becomes calcified to form cementum.
The first-formed cementum is acellular. Induced by the cementum, connective tissue fibres develop and transform into Sharpey’s fibres, the ends of which are embedded on one side in the cementum and on the other side in the alveolar bone. These fibres form the periodontal ligament that attaches the tooth to the alveolar bone. Cementum produced afterwards contains interconnected cells (cellular cementum). The production of cementum is a life-long process.
The Pulp
The odontoblasts are located in a neatly ordered fashion along the predentine. Dentine production continues steadily after the eruption (secondary dentine), although slowly. External stimuli, such as caries, evoke tertiary/reparative dentine formation (Chapters 5 and 8). The contents of the pulp include nerves, blood vessels and connective tissue.
3.2 Developmental and Acquired Structural Anomalies of the Enamel
Anomalies in the enamel structure may arise during enamel matrix formation or its resorption and subsequent calcification. Hypoplasia is a quantitative developmental defect caused by failure of matrix production or insufficient deposition of proteins on the outside of the developing enamel surface, whereby the normally smooth enamel surface becomes pitted or lacks in substance in large parts – it may be very thin or totally absent. Hypocalcification is a qualitative developmental deficiency that arises due to interruption of the resorption of the organic enamel matrix or a deficiency in the active calcium transport through the ameloblasts, and/or failure of maturation.
In both conditions the enamel is imperfect, but the term (hereditary) amelogenesis imperfecta is reserved for a group of inherited enamel abnormalities that occur in the absence of other tissue or systemic anomalies999 (and as a phenotypical feature in some syndromes, for instance with renal calcification),1032 although an association with biochemical changes elsewhere in the body may exist.21 When the enamel of a solitary tooth or a few adjacent teeth exhibits structural anomalies, a local cause is likely. A temporary systemic disturbance will lead to a chronologically linked disturbance in enamel formation of the (homologous) teeth formed at that time. More generalised defective structure of the enamel is due to systemic causes which are active during the whole period of tooth formation.
Appearance
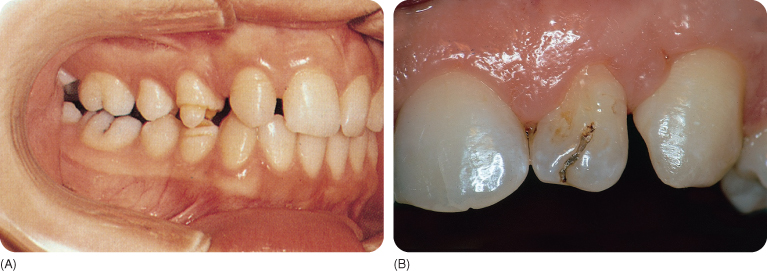
Hypoplasia is manifested locally as reduced enamel thickness, occasionally as pits arranged in rows and columns, especially in the buccal mid-third crown region235 326 327 of the permanent incisors and first molars (the lower canines in Western India).538 The defects may be microscopic in size.315 In more severe cases, the enamel is missing in some areas of the tooth (Figure 3.3).
Figure 3.3 (A) Hypoplasia of the enamel (attributed to tuberculosis). (B) Hypoplasia due to trauma of the deciduous predecessor leading to its intrusion.
Hypocalcification presents as localised abnormalities in tooth colour, called opacities, which may be:
- Diffuse, non-demarcated chalky-white spots
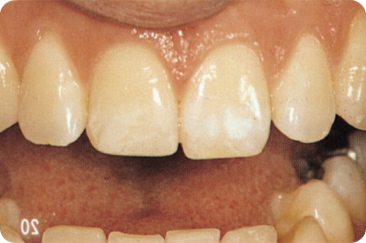
- Well-demarcated spots, often white (Figure 3.4), and otherwise cream, yellow or brown.
Figure 3.4 Hypocalcification (opacities) of the enamel of the upper central incisors.
The diffuse opacities are often less than 150 µm deep. The depth of demarcated areas varies. The white “spots” are softer547 than normal, translucent enamel, and the coloured opacities are even softer.857
The defects represent events in earlier life and may testify to fetal insults.438 Because the ameloblasts cease to exist after completion of amelogenesis and the enamel lacks blood vessels and an active metabolism, natural repair cannot take place. With time, the lesions may undergo alterations and tooth wear might make them less obvious.2
Prevalence
The prevalence of hypoplasia and hypocalcification varies widely from country to country and from time to time. For instance, children born in Sweden in 1970 showed more opacities in the deciduous dentition than those born before and after that year.471 Operationalisation of the definition of hypocalcification and hypoplasia is difficult. A carious “white spot” lesion and a developmental opacity may be diagnostically mixed up.346 963 1031
Opacities
The percentages of opacities in the permanent dentition varies between different countries, regions and towns from 30% to 75%:50 168 179 234 262 471 619 842 854 858 99% of 12-year-old Hong Kong children461 versus 2% of rural Chinese518 have been found to be affected. A few to some 20% of the deciduous dentitions show opacities.471 619 628 In an Irish study, 37–52% of cases had demarcated and 11–23% had diffuse opacities.248
Hypoplasia
Among 9–21 year-olds, 8–15% possessed hypoplastic enamel but reported rates for different populations70 234 248 461 518 531 755 628 919 vary tremendously – even up to 100%,718 whereas one small study reported a rate of only 3%.692
In the period AD950–1300 the proportion of hypoplasia increased in the Illinois area of the USA from 45% to 80%, in association with a change in lifestyle (with a decreased life expectancy) from hunting to agricultural-based settlements.327
Hypocalcification Index
In 1982, the Féderation Dentaire Internationale proposed the Developmental Defects of dental Enamel index (DDE)178 179 to quantify the type, number and localisation of the defects (but not their aetiology, which in many instances cannot be determined).178 According to the DDE index, a mean of 7.5–12.7 teeth/person have such defects.179
Causes
More than 90 causes of structural enamel defects have been noted, divided into local, systemic and inherited ones,404 for example, genetic abnormalities and syndromes, hormones, medicines, and infection and allergy.404 The presentation of enamel hypoplasia is rarely cause-specific. The location of the defects and distribution over the dentition are indicative of time the disturbance occurred (see chronology in Appendix I), which helps to determine the cause and predict which of the, if any, unerupted teeth will be affected. For example, the deciduous teeth and permanent incisors/first molars show signs of fetal and postnatal insults.
The calcification times (see Appendix I), determined with the help of radiographs, are possibly underestimations. Examination of fetal material has showed the initiation of calcification to occur earlier, in the twelfth fetal week477 or between week 5 and 13.870 The age of fetuses is difficult to ascertain and lethal conditions possibly influenced the calcification times.
The appearance of opacities also is not cause-specific. The enamel organ responds to the wide spectrum of insults in a limited number of ways and the threshold levels of many aetiological agents are unknown. The aetiology may therefore be difficult to determine, more so in the case of diffuse opacities.797 One main cause of enamel opacities is too much fluoride (Section 3.2.5), but other causes also underlie more or less clinically identical defects.
A low serum calcium level would explain hypoplastic enamel and a deficiency in phosphate the presence of a large amount of interglobular dentine,292 635 but other mechanisms also exist. For instance: non-degraded amelogenins attached to the apatite crystals prevent their growth, but the affinity of the later-formed amelogenins for the crystals is diminished due to their lower molecular weight.133 499 518 825 In cystic fibrosis (see below), the disturbed pH regulation affects amelogenin processing negatively, causing defects in enamel structure. Fluoride does not affect the uptake of calcium from the diet, but inhibits enzymatic degradation of the amelogenins and affects the ameloblasts.45
3.2.1 Pre- and Perinatal Causes
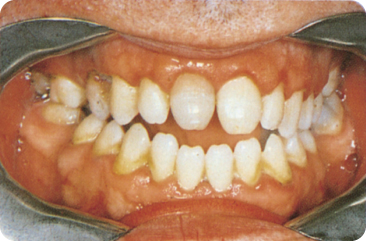
Incisor-Molar-Hypomineralisation (IMH; “Cheese Molars”)
This condition occurs when one or more first permanent molars show occlusal or larger areas of demarcated yellow to brown hypocalcified enamel. In the past they were called cheese molars because their colour and consistency resemble aged Dutch cheese. Post-eruptively, the enamel wears rapidly. The more yellow-brown the colour, the more porous is the enamel.426 The anomaly is named molar-incisor-hypomineralisation960 because the permanent incisors may be involved, the maxillary incisors more often than the mandibular incisors (Figure 3.5). IMH is, however, also observed in deciduous second molars, permanent second molars and the canines.958 A disturbance in enamel maturation is suspected,957 and the enamel is hypersensitive to cold stimuli.
Figure 3.5 (A–C) Incisors in molar-incisor-hypomineralisation (A), molars in molar-incisor-hypomineralisation (B) and molars (of another patient) in molar-incisor-hypomineralisation (C).
(Courtesy of K. Weerheim.)
No change in the function of the more cervically located ameloblasts indicates a temporary insult.426 The causes of hypocalcification include many well-known systemic health problems that occur around and up to 3 years after birth,911 1033 such as high fever, digestive tract disorders910 and problems related to birth (oxygen deficiency), and respiratory diseases427 911 such as asthma and bronchitis446 (the latter has been found to occur in regions with drinking water containing higher levels of fluoride).856 Other causes include: renal insufficiency, hypoparathyroidism, dioxins, diarrhoea and malabsorption.446 IMH in two siblings was ascribed to dehydration due to an intolerance of cow milk. Duration of breast-feeding might be associated with IMH,427 but birthweight and length at birth, problems around and during birth do not seem to be associated.92 426 IMH is seen in about 5 to >10% of children.220 428 446 470 959 A more extreme range has also been reported: from 3.5% to 25%.956 957
Small affected areas can be treated with fissure sealants. Somewhat larger areas may be restored with glass ionomer cement. Composite resins seem better,272 but the restoration fails frequently,1033 although crowns with two sound surfaces on follow up after 4 years were still satisfactorily restored (one-third remaining hypersensitive for 1 week and a few teeth for 1 year).539 The hypomineralised enamel shows unusual etching patterns, either because the defective enamel is not uniformly removed,547 or because of deviations in the prism boundaries857 or because the tissue underneath the sound enamel may be hypocalcified.426 428 Larger affected areas require onlays, stainless steel crowns,272 or adhesive copings.1023 To avoid a repeated cycle of restorations, extraction of a severely affected first molar should be considered,985 ideally at age 8–9 years, when radiographs show complete calcification of the crown of the second molar or when its bifurcation is visible, which minimises the need for orthodontics. Following late extraction of the first molar, the second molar will show less forward movement, with mesial tipping and lingual rolling.986 If the first molar is extracted too early, the second premolar will drift distally. To prevent a centre line shift, a non-compromised contralateral molar may be removed too.986
Congenital Syphilis
Intra-uterine infection with the spirochaete Treponema pallidum causes among other defects, deafness, blindness and a typical enamel hypoplasia: the “triad of Hutchinson” (English physician, 1820–1913).
Evidence of venereal syphilis in European skeletons post dates Columbus’ discovery of the New World, but the disease has been noted in skeletons in the Dominican Republic from the pre-Columbian time, which is why it is considered that the disease spread from the New World.746
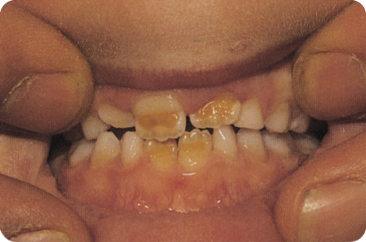
Hutchinson’s incisors are small and barrel shaped. They are called “screwdriver teeth” because the approximal surfaces converge towards the incisal, the vertical middle third of the labial surface being absent. The incisal edge shows two mamelons flanking a deep crescentic notch (Figure 3.6). Only two pulp horns are present. Alterations in blood vessel walls, oedema and degeneration of the centrally situated ameloblasts in the papilla account for the decreased central growth. Wear of the dirty-greyish incisors subsequently eliminates the notch.122 208 391 A hypoplastic, incisal constriction in the permanent canines is not likely to be “syphlytic”.746
Figure 3.6 Screwdriver teeth in congenital syphilis. Note the characteristic central notches in the lower incisors.
(Courtesy of Department of Oral Surgery, University of Groningen.)
The rounded permanent first molars with a large number of small occlusal cusps, with pigmented areas in between, resemble a mulberry (“mulberry molars”, “Moon’s molars” after Henry Moon). Parts of the enamel may break away.391 Hutchinson’s triad is fully present in 1% of the patients: about 30% have screwdriver teeth and mulberry molars.122 283 391 706 For unknown reasons the incisors are not always bilaterally anomalous. The deciduous dentition is free from defects: the spirochaete does not penetrate the tooth germs before the fourth (or fifth) month in utero, when the placental Langerhans layer disappears,941 but crown growth might be disrupted after birth (unlikely) rather than before.391
Owing to the inadequate treatment alternatives prior to the Second World War, almost all incisors and first molars in such cases were hypoplastic.564 From 1943, syphilis declined,932 but its incidence increased again around 1960,283 941 a trend that has persevered;605 it continues to be seen in the USA and Europe especially among males not using condoms, who are at a higher risk.489 Antibiotics control the disease effectively. If left untreated, the primary effects disappear spontaneously, but the patient enters the next stage(s) of the disease.
Though the dental features are considered to be distinctive,391 the diagnosis of syphilis cannot be based on the morphological features of the teeth, which are also seen in Nance–Horan syndrome.330 658 The microorganisms must be demonstrated, and serological examination should show an increase in the titre of antibodies.
Cystic Fibrosis
This is an inherited pancreatic disease, which affects the chloride channel that regulates salt and water excretion in exocrine cells. Both cystic fibrosis and long-term administration of tetracyclines for airway infections are associated with disturbances in enamel formation; they are described later in the chapter.
Cytomegalovirus (CMV)
This virus is a member of the herpesviruses group and causes severe morbidity congenitally (e.g. pneumonitis). Intra-uterine infection, in 0.3% or 2%,607 1021 affects the DNA, but 90% of the children are asymptomatic.1021 Of prematurely born infants, 13% are infected.238 If moderate, mucosal lesions mimic other ulcerations (e.g. aphthous ulcers, herpetic stomatitis).105 The more severe the infection, the more severe are the dental anomalies, ranging from opaque deciduous enamel to yellow discoloration and considerable hypoplasia.102 Hand washing by pregnant women and the patients’ caregivers effectively prevents acquisition of the virus.659 1021
Gestational Diabetes
Diabetic mothers may give birth prematurely in the 36–37th week or before, or deliver a child by caesarean section, who is often overweight.339 Deficient insulin production (type 1) alters the metabolism of carbohydrates (hyperglycaemia with glycosuria), proteins and lipids. In type 1 diabetes, the body makes antibodies against the Langerhans cells in the pancreas: less and less insulin is produced. Uncontrolled diabetes causes among other conditions, vascular and kidney disease and blindness.819 Tongue burning and Candida infection are also likely.
Children of diabetic women show deciduous enamel hypoplasia more often than other children,337 about 40% versus 15%, respectively.339 The enamel matrix amelogenins are insufficiently resorbed,648 817 even after insulin supplementation. Children born prematurely have a broad neonatal line in the permanent teeth and in 77% the postnatal subsurface enamel is hypomineralised.638
The neonatal line is found in teeth that start to develop in utero, the deciduous teeth and the permanent first molars and central incisors,991 and is a lasting testimony of the environmental changes at the time of birth.774 980 990 Enamel prisms crossing the line have an altered orientation and are less densely packed in postnatal enamel.981 Neonatal hypocalcaemia in the children may be the result of fetal hypoparathyroidism (Section 3.1.5).641 902 A high proportion show hypocalcified permanent incisors despite correction of the hypocalcaemia.691 However, every neonate has some hypocalcaemia,851 which is corrected on breast-feeding (which influences also mandibular growth).969 In children of diabetic mothers the calcium content at birth is comparatively low and later it decreases more than in others.
Breast milk contains too little calcium and phosphorus for pre-term children, but cow’s milk cannot be given because of its high phosphate level, which further increases the hypocalcaemia between days 2 and 5.318 Children with clinical signs of neonatal hypocalcaemia may show a groove in the enamel surface instead of the neonatal line,508 more so when the hypocalcaemia is severe and of longer duration.
Dioxins
High dioxin concentrations in breast milk owing to environmental contamination (until 1987) caused enamel hypomineralisation and hypoplasia and dentinal defects in the permanent first molars and incisors of many children.13 No child with enamel defects in a sample of Finnish children had been breast-fed for less than 8 months.14 15 Dioxins are by-products of the manufacture of phenols and combustion of chlorine-containing waste. Near such factories, dioxins may be present in cow’s milk and in pigs due to food pollution. After an explosion in one chemical factory, the 5-year-olds who lived nearer the factory and therefore had greater exposure to dioxin were more likely to have hypoplastic enamel.13 Environmental dioxin levels (cow’s milk) currently are probably of minor or no account with regard to dental defects, but little is known about synergistic effects with other chemicals.1034
The placenta transports dioxins. High concentrations have been found in adipose tissue of children who died in the early neonatal period.474 The most toxic chemical compound in the dioxin group caused depolarisation of odontoblasts and ameloblasts in cultured embryonic molars. The dentine matrix failed to mineralise and the enamel matrix was not deposited. Dioxin affects epidermal growth factor receptor (EGFR) signalling and thereby odontogenesis.677
Erythroblastosis Fetalis and ABO Incompatibility
Congenital haemolytic anaemia (erythrocyte destruction) is caused by the presence of incompatible proteins in the cell membrane of the fetal and maternal blood cells: the Rhesus factor (Rh) (the rhesus monkey possesses the same factor).
When a Rhesus negative (Rh−) woman (probability 15%) becomes pregnant from a Rhesus positive (Rh+) man (probability 85%), the fetus has Rh+ blood, unless the father is heterozygote (which occurs mostly): then the child has a probability of 50% of having Rh+ blood.809 Cells from the child (antigens) leak into the mother’s blood at term and during delivery. Antibodies developed in the mother cross the placenta during the next pregnancy, whereupon the second fetus develops haemolytic anaemia with hyperbilirubinaemia, manifested as jaundice (formerly called icterus gravis neonatorum).
For neonatal jaundice appearing between day 4 and day 7 of life, breast milk is the most common cause.78
The haemolytic product bilirubin (e.g. porphyrin remnants after degradation of the erythrocytes) and its oxidation product biliverdin circulate in the blood. The child’s liver cannot process large amounts of bilirubin, which is therefore deposited in the body tissues. Bilirubin causes jaundice, damages the basal ganglia in the brain (kernicterus) and discolours the dentine. Jaundice is not a disease but a symptom and is treated by exposing the child to a blue – nowadays a green – light, which transforms the bilirubin into a form that the child’s liver can process.
If the Rh− mother has not been treated with immunoprophylaxis, Rh+ blood needs to be transfused to the second and any other children. Only 1 : 200 neonates experience the above symptoms,92 809 because not all mothers form antibodies, and the antibodies do not always cross the placenta and immunisation could occur.809
Structural Anomalies
About 0.1% to 30% show enamel hypoplasia in the teeth formed at birth.56 383 692 Tooth germs in stillborn and deceased neonates demonstrate vacuoles in the regions of the odontoblasts and ameloblasts, corresponding to structural defects in the permanent teeth.383 The hypoplasia depends on the albumin to bilirubin ratio; the amount of unconjugated bilirubin determining the level of damage to the ameloblasts.56 Rh− incompatibility may also affect the dermatoglyphs of the hands and feet.56
Discoloration
In hyperbilirubinaemia, soft tissue turnover removes the bilirubin deposited throughout the body, but it remains semi-permanently entrapped in the teeth.952 Tooth discoloration is also possible without signs of kernicterus.971 Bilirubin in the dentine discolours the teeth in 10–70% of the deciduous dentitions green (incorrectly called “chlorodontia”),952 972 blue, grey, yellow and brown.329 383 563 The discoloration disappears spontaneously after some years.692 779 809
Bilirubin (as a green line parallel to the incremental lines) has been found also in the deciduous teeth of patients with a history of severe liver dysfunction.952
ABO Incompatibility
The erythrocytes contain the blood group antigens A, B, AB or O. During pregnancy, a woman may become sensitised against a blood group antigen present on the child’s erythrocytes. The mother’s IgG anti-A and anti-B antibodies (not the IgM antibodies) cross the placenta. Depending on the mother’s IgG titres,907 a severe haemolytic reaction follows, though less often than in erythroblastosis fetalis.383 Symptoms include anaemia due to excessive destruction of the erythrocytes, kernicterus and icterus gravis. In children of A and B blood group mothers, the incidence of significant hyperbilirubinaemia is not increased.671 Children of O blood group mothers and non-O fathers have a 5–10 times increased chance of needing exchange transfusion due to hyperbilirubinaemia,372 906 in addition to treatment with phototherapy. Haemolysis is seen in 0.03%.575
Serum bilirubin measurement at the sixth hour of life is used to predict whether a newborn will develop hyperbilirubinaemia (critical level 4 mg/dL) and/or a haemolytic reaction (critical level 6 mg/dL).767
Reports of dental effects of ABO incompatibility are lacking, except one case of discoloured deciduous anterior teeth.77 Most children receiving blood transfusion after birth have hypocalcaemia for at least 3 days. Their deciduous dentition shows hypoplasia and hypocalcification near the neonatal line.715
Infantile Encephalopathy (Central Paralysis, Cerebral Palsy, Other Neurological Disorders)
Improved neonatal care has enlarged the numbers of surviving, prematurely born children and the survival of those with brain damage through asphyxia (deficient oxygen) or ischaemia (insufficient blood). Larger numbers of children with delayed psychomotor development and, later, spasticity now survive. The probability of development of cerebral palsy, a motor disorder caused by brain damage, increases with antenatal complications, such as infection of extra-embryonic membranes, and delays in caesarean section decisions (unless carried out at the expected delivery time).616
Cerebral palsy and enamel hypoplasia are associated,315 but many of these children experience other pre- or perinatal disturbances such as asphyxia and fever,438 which may be the real cause of the enamel defects. The likelihood of hypoplastic deciduous enamel increases if premature babies have cerebral palsy:600 in one report about 30% had enamel pits, vertical or horizontal grooves or coloured enamel opacities102 and 45% had enamel hypoplasia when cerebral palsy occurred in combination with hereditary developmental defects of the central nervous system.475
In a study of mentally retarded children, almost half of the central incisors exhibited isolated white opacities and more than one-quarter of the teeth had several white diffuse opacities; these children had suffered significantly more bacterial diseases than other children.566 Children with minimal brain dysfunction show a broad neonatal line and hypocalcified prenatal enamel,640 but Rh incompatibility could also be responsible.4 565 919 920
The prevalence of hypoplasia varies in reports from 0% to 35% and 68%.565 600 919 The children’s age at the time of the study may be a factor. In a follow-up study, the number of hypoplastic lesions reduced within 6 months, owing to wear, fractures and so on.103 Inconsistent associations may be a result of methodologically limitations of older studies.102
Intolerance to Cow’s Milk
Non-allergic hypersensitivity to cow’s milk affects amelogenesis, by interfering with the activity of vitamin D (Section 3.2.3) and leading to calcium and phosphate deficiency.794
Hypoxia
Maternal or neonatal hypoxia (oxygen deficiency) causes defects in the enamel structure and is closely related to prematurity. The occurrence of enamel defects was similar in children with and without history of neonatal asphyxia,341 but later a significant difference was found.342 433
Central cyanosis and hypoxia was noted to result from transposition of the great arteries, in 1 : 5000 births, and may recur after surgical treatment.610 Suffocation almost to death in experimental animals influenced the structure of the enamel.921
Orotracheal Intubation
An orotracheal tube used for oxygen administration to premature babies was excluded as a cause of incisor damage,433 but there are indications that the tube, by compressing the alveolar processes,176 is associated with enamel hypoplasia in 50–95% of children undergoing tube placement. The deciduous and permanent incisors particularly on the right side are affected, due to the securing of the tube.39 Laryngoscopy causes defects on the left side,802 but a right-left difference has not always been reported.268 The longer the treatment, the greater the number of maxillary deciduous teeth that show hypoplasia.10 Preterm children with osteopenia (undermineralised bone) may require laryngoscopy.803 Intubation with a tube pressing on the alveolar bone may lead to dilaceration.39
The teeth are yellow-brown,268 and opacities are observed outside the area affected by the tube, e.g. in deciduous first and second molars,801 and also in cases with perinatal hypoxia.754
Errors of Metabolism
Phenylketonuria
Chiefly seen in Caucasians, this is an inherited absence of a liver enzyme (phenylalanine-hydroxylase) or a co-enzyme (apo-enzyme) that inhibits the transformation of the amino acid phenylalanine into tyrosine and phenylalanine accumulates.167 937 Without dietary therapy, a consequence is brain damage (mental retardation, convulsions). The disorder may cause enamel hypoplasia and brown discoloration.
Ochronosis
An inheritable deficiency in the enzyme homogentisate oxidase underlies the faulty degradation of phenylalanine and tyrosine.693 Consequently, homogentisine accumulates in the body causing ochronosis. The colour of the connective tissues and teeth is blue to black,693 and occasionally dark brown, and the enamel might be hypoplastic.
Galactosaemia
Galactosaemia occurs when the galactose metabolism through absence of enzyme activity (galactose-1-phosphate-uridyl-transferase) results in hepatocellular injury and developmental delay.94 Continued consumption of galactose and lactose (milk) in early childhood leads to enamel hypoplasia in the permanent teeth.94 Mild untreated galactosaemia does not interfere with amelogenesis. A subsequently severe course is associated with hypoplasia of the enamel formed at that time.
Rubella (German Measles/Rubeola) during Pregnancy
The neonate of a mother affected with the viral disease rubella before week 13 of pregnancy348 has many congenital disorders due to transplacental transfer of the infection. The virus can be demonstrated until 3 years after birth.348 General developmental abnormalities include abnormalities of heart, eyes, ears, brain, bone, low birthweight,615 and even death may occur.102 Congenital dysfunction of the salivary glands1022 promotes caries and tooth wear.
Some decades ago, tooth defects were not viewed to be associated with rubella. In the absence of rash in the mother (pink macular exanthema), the disease was overlooked. Grahnén in 1958 concluded that it was not possible to assess whether the disease had dental consequences,336 but later a relationship was established.348 349 Retrospectively, 82% of children with rubella embryopathy showed enamel defects against 9% of controls.356 The eruption is delayed, which is not associated with either birthweight or length at birth.535
Segmental Odontomaxillary Dysplasia (Chapter 2)*
In this condition, the deciduous molars are hypoplastic or atypical.51 91 228 305 597 964 In cases of hypodontia,216 330 the eruption is delayed or teeth remain unerupted.91 201 597 974 Unusual external resorption of such deciduous molars has been reported.51 91 672 703 The pulp shows fibrous enlargement and a deficient odontoblastic layer, and there are tubular defects in the coronal dentine.51
Sickle Cell Anaemia
This disease belongs to a group of autosomal recessive inherited disorders of haemoglobin formation with icterus, and occurs almost exclusively in persons of African origin and in the south and east Mediterranean regions. The relatively short-living red blood cells are sickle shaped.
In haemoglobin, haem is coupled to polypeptide chains of the globulins α, β, γ and δ. In sickle cell anaemia, β-globulin is chemically abnormal (while in β-thalassaemia, β-globulin is normal but formed in insufficient amounts although the other globulins are increased). In heterozygotes, periods with few complaints alternate with periods of sickle cell crisis. In homozygotes the disease is fatal.204 Patients with the “Mediterranean disease” (thalassaemia major) usually require blood transfusion to normalise their haemoglobin level in order to prevent hypoxia. The transfusions may lead to iron overload and haemosiderosis, causing morbidity and mortality. Instead of management with transfusions, bone marrow may be transplanted.369
Sickle cell anaemia is associated with hypocalcified enamel and accentuated perikymata, poorly mineralised dentine, obstructions in the dentinal tubules, denticles, and hypercementosis.831 The size of the opaque crowns and roots is reduced.369
Vitamin D Deficiency in Pregnancy
Vitamin D deficiency in the mother influences pre- and perinatal amelogenesis.705 822 The deficiency leads to maternal hyperparathyroidism, which suppresses fetal parathormone production (Section 3.2.4).705 The baby shows signs of tetany (tonic and clonic muscle spasms) due to hypocalcaemia. Enamel hypoplasia develops postnatally, due to the transiently inactive ameloblasts.508
Weight at Birth and Preterm Children
Low birthweight, in particular in prematurely born children, is associated with enamel defects,273 338 518 743 and intubation and hypoxia may be responsible.268 273 295 433 Failing postnatal (intravenous) feeding, hypocalcaemia and systemic disturbances that underlie the low weight are co-factors.273 518 637 801 796 The relationship between prematurity, hypocalcaemia and hypoplastic enamel appeared insignificant in one report,691 but these conditions all have mineral deficiencies in common.796 Children with a low birthweight may show subnormal growth934 and develop idiopathic epilepsy more often than others, along with hypoplastic, orange-coloured enamel.580 581
In 1990, 7% of the neonates in the USA weighed <2500 g due to reasons including maternal smoking.934 935 Almost all neonates weighing 2000–2500 g, 50% of those weighing 700–800 g and 10% of those weighing 500 g survive.796 936 The sequelae of a very low birthweight include long-term general health problems, poor physical growth, behavioural disorders, etc.936 Low birthweight children consume less maternal milk in the first weeks after birth; the few but more severe dental defects are worsened by seasonal deficiencies in calcium and vitamin D.588
Discoloration
A few cases show horizontal yellow bands in the deciduous teeth, presumably due to a high bilirubin serum level or a low calcium level.295 Opacities are less common than hypoplasia.802
Enamel Defects
A high proportion of symmetrical enamel hypoplasia cases are associated with low birthweight.102 841 Generalised hypoplasia affects 40–70% of the deciduous dentition796 and 10–20% of the permanent first incisors and molars.273 295 518 794 795 The high prevalences have been corroborated by other studies,356 518 842 for children with and without kernicterus.600 Prematurity (odds ratio 2.6) and no breast-feeding (odds ratio 3.2) are associated with developmental defects in the enamel of primary teeth.
In spite of supplementation of breast milk with minerals and vitamin D, in one study enamel structure was impaired in 84% of preterm children (weight <2000 , gestational age <37 weeks), versus 36% in controls.10 The lower the birthweight, the higher the frequency, especially of hypoplasia.802 In the absence of macroscopic anomalies, the enamel contains microscopic pits.804
The deciduous molars of very low birthweight children, in particular, in extremely low birthweight infants, are comparable with those of controls who are smaller in size.296
Other Conditions
Several conditions, such as nephrosis, spina bifida, meningitis and dermal disorders are incidentally reported to be associated with structurally deficient enamel. Half of children with congenital cardiovascular disorders (patent ductus arteriosis, cardiac failure) are reported to have deciduous enamel hypoplasia.357 796 Stillborn children and neonates dying upon birth have more or less severely anomalous enamel.478 In defects of the biliary ducts (1 : 10 000–14 000) and end-stage liver disease, the green discoloured postnatal deciduous enamel shows hypoplasia.805 1024 Congenital allergic conditions, such as asthma,722 affect the enamel structure.919 Haemangiomas of the lip may indirectly cause hypoplastic enamel, but this hypothesis has not been confirmed.58
Curvilinear hypoplasia on especially the labial surfaces of the deciduous mandibular canines is present in one-third of (African American?) children in Mississippi, USA.236 Local thinned cortical bone is assumed to protect insufficiently against minor injuries.796
It has been hypothesised that anomalies of both the brain and the enamel may have identical causes. But learning disabilities at school were found to be unlikely to be associated with the hypoplastic enamel of deciduous teeth.509
3.2.2 Postnatal Infectious Diseases
Several infectious diseases cause structural enamel anomalies.822
Exanthematous Diseases
The viral infectious exanthematous diseases such as measles have been stated to cause hypoplastic enamel,271 but most seem at the most associated with it.422 873 A causal relationship is unlikely, but a very sick child with minimal resistance might develop dental hypoplasia.692 There is no evidence of an effect of the exanthematous diseases on the teeth,292 338 422 535 693 858 982 except for rubella, high fever lasting for longer periods and varicella infection in the third year of life.422 883 A short bout of high fever will not affect the ameloblasts that are resting at that time, which might explain why some children are free from enamel anomalies and others are not. In some studies, the majority of children had an exanthematous disease after the enamel anomalies had already developed.422 982
Cells damaged by viral diseases are replaced by proliferating neighbour cells.941 Skin eruptions, for instance, in measles, are for a large part allergic in nature. In how far other tissues of epithelial origin are directly or indirectly affected by viruses, is not known.
Other Postnatal Infectious Diseases
Non-exanthematous infectious diseases affect amelogenesis: whooping cough, pneumonia,836 tuberculosis, diphtheria and gastrointestinal illness317 826 883 with diarrhoea that disturbs the calcium metabolism and causes dehydration, vitamin D deficiency,102 292 urinary tract infections,858 883 982 especially in the second year of life,883 otitis media842 in the third year of life,883 convulsions982 and malaria (Figure 3.7).
Figure 3.7 Malarial fever was assumed to have resulted in the hypoplasia and brown discoloration of the enamel of the permanent central incisors seen here. The permanent first molars were likewise affected.
The relationship between diarrhoea (Salmonella infection) and hypoplasia requires confirmation.826 Parasitic diarrhoea was found to cause enamel hypoplasia in animals, attributed to hypocalcaemia. The ameloblasts showed vacuoles.855
These diseases have in common high fever with dehydration, which causes inadequate kidney function, and which in turn alters the calcium : phosphate ratio of serum. Diseases of the kidneys, such as “nephrotic syndrome”423 653 814 are associated with enamel hypoplasia. The tooth defects may also be caused by medicines such as the tetracyclines.
3.2.3 Malnutrition and Systemic-Nutritional Disorders
A sufficient supply of calcium and phosphate, the building blocks of hydroxyapatite (Ca10(PO4)6(OH)2), and an adequate nutritional supply of vitamins and trace elements are essential for enamel and dentine matrix formation.
Nutritional Deficiencies
Severe and chronic malnutrition causes (chronological) enamel hypoplasia,102 256 415 750 751 874 with black stained teeth.770 The substandard diet and health status in children and adolescents whose remains were excavated from a sixteenth- to eighteenth-century graveyard in London had caused severe enamel hypoplasia and molars with multiple additional cusps (resembling the mulberry molars).1035 White or otherwise discoloured band-like constrictions encircling the deciduous incisors, called odontoclasia, were more frequent and severe whenever malnutrition was more evident. The occurrence of enamel hypoplasia and hypocalcification has been found to be race-related/dependent, as is malnutrition.361 362 Nutritional status is a factor, but birthweight and enteropathy and child’s stature531 seem of more importance (Table 3.1).751
Table 3.1 Factors related to the prevalence of developmental enamel defects in deciduous teeth751
| Cause | Risk factor | Odds ratio |
| Generalised defects | ||
| Birthweight | <2.5 kg | 5.0 |
| Enteropathy | Disease | 3.7 |
| Oral trauma | Trauma | 3.0 |
| Nutritional status | Measured with height for age | 2.6 |
| Toothpaste swallowed | 2.4 | |
| Area | Urban low class-rural low class | 2.2 |
| Bronchial disease | Disease | 2.0 |
| Distinct defects | ||
| Birthweight | <2.5 kg | 3.0 |
| Nutritional status | Measured with height for age | 2.8 |
| Bronchial disease | Disease | 2.5 |
| Hypoplastic defects | ||
| Birthweight | <2.5 kg | 2.2 |
| Nutritional status | Measured with height for age | 1.9 |
The supply, for instance, of vitamins will also continue to be low in malnourishment.874 Among a sample of severely malnourished children, 73% had hypoplastic deciduous incisors versus 43% with moderate malnutrition.874 Of less well-fed children, about 20% showed hypoplasia against none with a good diet.256 Similar results were found in another study.586 770
Minerals
Calcium
The dry weight of calcium in the enamel is 34–40% and in the dentine 26–28%. To affect the teeth, the calcium deficiency must be extreme.193 The combined action of parathormone, calcitonin and vitamin D maintains very stable calcium and phosphate blood levels, even when the mineral intake is suboptimal. When the bone shows signs of deficiency, the enamel will be normal, but the dentine seems more vulnerable.
At birth, the calcium level suddenly decreases. A very low level, 7.5 mg/100 mL and less (at which neonates experience tetany) is associated with enamel hypoplasia and abundant interglobular dentine.777 An experimental study found that diet-induced chronic hypocalcaemia in rats probably interfered with cellular and extracellular events during enamel maturation,624 and caused hypocalcification of the enamel of the incisors, with many residual enamel proteins.116 While human teeth are formed in a different way, dietary hypocalcaemia may affect development of human enamel.
Pregnancy and lactation alter the maternal skeletal metabolism to accommodate fetal skeletal mineralisation and milk production.668 Rats nursed and weaned after 30 days on a diet low in calcium had hypocalcified enamel. Pups fed the first 10 days on a normal and the next 20 days on a low calcium diet had normally calcified enamel.116 In a similar experiment, low birthweight offspring were weaned on a calcium-free diet: the enamel matrix was hypocalcified and the reduced dentine matrix was normally calcified. When restored to a calcium-containing diet, the enamel became completely mineralised and the dentine thickness and mineral apposition increased.527 A diet low in calcium caused variable and heterogeneous distribution of amelogenins in the enamel, with albumin leakage into the enamel organ.624
Phosphate
The dry weight of phosphate in the enamel is 16–18% and 12% in the dentine. Phosphate deficiency is commonly not due to insufficient supply, but due to other causes, such as vitamin D-resistant rickets.719
Trace Elements
Magnesium (0.4% dry weight of the dentine and the enamel)777 appears essential in odontogenesis,407 but teeth with higher levels of magnesium are more vulnerable to caries.777 Insufficient iron causes enamel hypoplasia through enzyme damage.701
Fluoride is also essential for odontogenesis.322 632 Other trace elements (which are not similarly distributed within the enamel and dentine) include: zinc, silica, and to a lesser degree copper, chromium, manganese, molybdenum and tin. Very small quantities of selenium, vanadium and cobalt are also present. Their importance in odontogenesis is largely unknown. Strontium in the crystal lattice may cause diffuse opacities.195 A diet may not contain all the above elements.
Proteins
Diets with variable ratios of sucrose and casein, fed to female rats during pregnancy and lactation, resulted in smaller molars (due to reduced amounts of dentine) in the offspring. When a diet low in protein was given, many of the offsprings’ third molars lacked cusps, that is, small amounts of protein deficiency at different times during the perinatal period resulted in abnormal cuspal patterns of the third molars.626
Protein deficiency in animals increases the risk of enamel hypoplasia874 and caries,46 642 and hypoplasia may enhance caries progression. Malnourished children, however, show hardly any dental caries, possibly because of the lack of sucrose in the diet. Prenatal malnutrition causes hypoplasia of the deciduous enamel, which was found to associated with caries development in Native Americans living in 100BC to AD500.183
Vitamins
The average diet in Western industrialised countries guarantees roughly an adequate supply of vitamins.240 384 Deficiencies are apparent in various groups including older people, vegetarians, alcoholics and persons with other addictions, immigrants, young people, ill or cancer patients, etc.240 778 780
Vitamins B and E are water-soluble, and A, D, K and E are fat-soluble. Excess amounts of B and C have a low potential for toxicity, because they are rapidly excreted with the urine.
Vitamin A
Vitamin A in its active form is present in meats and as pre-vitamin in vegetables and fruit. The vitamin is stored in the liver and is mobilised from there, and is essential for embryogenesis, growth and epithelial differentiation. Deficiency of vitamin A, hypovitaminosis A, causes night blindness, and if this is long-lasting, corneal keratinisation develops, with furthers alterations in the mucosa and the brain. Severe hypovitaminosis A causes disturbances in the formation of the (pre)dentine and enamel (matrix).366 586
Hypovitaminosis A was found to alter collagen synthesis and the calcium : phosphate ratio in molar tooth germs in rats. The ameloblasts atrophied and dentinogenesis was delayed.627 Hypovitaminosis A during the first postnatal month resulted in pitted enamel in many rats.873 Infants with hypovitaminosis A were found to have atrophied tooth germs. In less severe cases, the stellate reticulum was condensed, the dentine was poorly mineralised, and the predentine was widened.221 Another study found that vitamin A-deficient rats lacked odontoblast differentiation, and retinoic acid supplementation reduced this effect.704
The incisors of rats fed for 200 days on a diet poor in vitamin A showed marked morphological changes; the enamel was folded in an accordion-like fashion.182 In another study, the inhibition of intra-uterine growth of the rat tooth germ due to vitamin A deficiency was transient, although the enamel remained less dense.290
Hypervitaminosis A, through its toxic effects, also impedes the growth and development of the tooth germ, differentiation of the odontoblasts and possibly the ameloblasts.157 410 Where odontoblasts are formed, predentine development is disrupted. Hypervitaminosis A also causes gingivitis, fissures at the angles of the mouth, and generalised symptoms (irritability, headache and fatigue).280
Injected radio-labelled vitamin A has been found to be taken up by the liver, kidneys and ameloblasts active at that time, but not by the hard tissues.818 These finding might be related to the decreased stratum intermedium volume in hypervitaminosis A.182
High-dose vitamin A supplementation in pregnant women results in fetal defects, although exact amounts are not known.745 967 A range of 700–1000 µg vitamin A/day has been advised; 3000 µg/day may be the upper limit in post-menopausal women to alleviate the risk of hip fractures,279 but this dose certainly seems too high for the unborn child.
Vitamin C
This vitamin is present as ascorbic acid in (plant) foods. Children up to the age of 3 years need 20 mg vitamin C/day, increasing to 30–35 mg/day in 7–9-year-olds. A large kiwi fruit contains about 70 mg of vitamin C.749 Hypovitaminosis C, also called scurvy, causes weakness, anaemia, subcutaneous and gingival bleeding and loss of teeth (scurvy was formerly fatal in sailors on long sea voyages).
Deficiency of vitamin C at the time of the odontogenesis causes pitted enamel and reduces the height of the odontoblasts, which also become disorganised. Bleeding is seen in the pulp and in the enamel organ.692 In vitro explanted/excised tooth germs appear to need vitamin C for the synthesis of collagen131 380 and the incorporation of proline (and lysine) into the collagen molecule.940
Vitamin D
This vitamin is formed from a provitamin (7-dehydrocholesterol) in the skin under the influence of sunlight. The vitamin is present in some foodstuffs as ergocalciferol or cholecalciferol and is transported to the liver and hydrolysed into 25(OH)D3, which is metabolised in the kidneys into the active, functional “vitamin D3 hormone”, i.e. 1,25 (OH)2D3. D3 hormone is of importance in the resorption of bone,552 but its role and that of the other vitamin D metabolites has not been fully elucidated,282 partly because the consequences of a deficiency are compensated for by normalisation of the plasma level of calcium,712 or a direct effect on ameloblasts.884
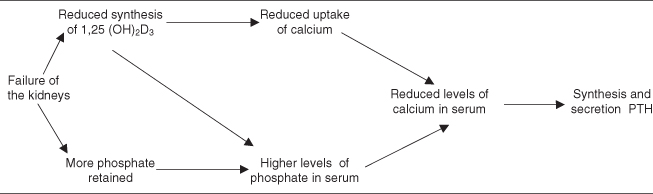
Vitamin D together with parathyroid hormone (Section 3.2.4) regulates the serum levels of calcium and phosphate, even when the supply is irregular. Hormonal D3 regulates the serum concentrations of calcium and phosphate, stimulates the uptake of these minerals in the small intestine, promotes the transport of calcium within the skeleton, and directs the reabsorption of calcium in the kidneys.282 Kidney malfunction reduces the excretion of phosphate, which causes hyperphosphataemia and formation of calcium–phosphate complexes, which in turn induce secondary hyperparathyroidism.708 Hypoplastic, hypocalcified and discoloured enamel and pathological root resorption is present in children with chronic renal failure.162 Severe chronic renal failure results in reduced synthesis of D3 hormone, which then leads to increase in the synthesis and secretion of parathormone; this is also a cause of secondary hyperparathyroidism (Figure 3.8).
Figure 3.8 Renal failure promotes the synthesis and secretion of parathormone (PTH).162
Hypovitaminosis D causes rickets in children; nowadays rickets is rarely seen in the developed Western countries, but it continues to occur in countries such as Yemen and Saudi Arabia243 906 where children’s and women’s skin is not exposed to the sun (even after emigration to countries in the temperate zones). Children born to mothers with vitamin D deficiency in sunny Crete (Greece) were found to respond favourably to treatment with calcium and vitamin D.30 Breast-fed children may have vitamin D deficiency if the mother is a strict vegetarian.
In rickets, the skeleton does not ossify completely and the osteoid tissues hypertrophy. Under normal loading, the bones become distorted, and the clinical features include lateral bowing of the legs and scoliosis. The dental hard tissues are affected, particularly the dentine: irregular tubules, atubular globular areas, and a large amount of interglobular dentine are evident.80 163 587 635 With more severe rickets, the defects noted in the teeth also increase.244 340
Steady-state levels of amelogenin and enamelin mRNA, which regulate the tooth proteins, were reduced in the incisor epithelium of vitamin D-deficient rats, which also showed a dramatic decrease of interprismatic enamel.675
Calcium deficiency is more serious than a reduced supply of vitamin D.587 Vitamin D deficiency alone has less impact on the teeth than on bone, but a combined deficiency of vitamin D and calcium affects the teeth and bones to the same extent.277 With such a deficient diet, the calcium serum levels are reduced,255 the predentine layer is widened255 due to accelerated protein production,252 254 and odontoblastic enzyme activities are also affected.252 255 256 455 However, the deficiency state must be sustained for a longer period to cause enamel defects.714 Whether the hypocalcaemia or the increased synthesis of parathormone enhances the activity of the odontoblasts is unknown.253 The secreting ameloblasts are also affected directly.885 Severe rickets is accompanied by significant enamel hypoplasia in some regions, but not in others.271 692 The pulp chambers and horns are large.635 Rachitic deciduous dentitions exfoliate early635 and children often show a vertical open bite.810
Calcium (900 g/day) and fluoride (50 mg NaF/day) supplements have been used to treat osteoporosis in post-menopausal women in whom oestrogen production has dropped. If not complemented by a huge supply of vitamin D (50 000 units/week), large amounts of unmineralised bone matrix are formed.437 Fluoride supplementation via milk (calcium) in vitamin D-deficient women does not alter the (subclinical) signs of rickets.845
Hypervitaminosis D is rare and leads to hypercalcaemia. Symptoms include calcium deposits in the kidney, weakness, gastrointestinal distress and deranged fat metabolism.280 Enamel hypoplasia (depressions) corresponds to the time of the hypervitaminosis. Dogs had hypermineralised enamel.320 692
Vitamin E
This vitamin stabilises the unsaturated fats against auto-oxidation, thus protecting the cellular membranes against damage. Vitamin E supplements are given to prematurely born children as prophylaxis against hyperbilirubinaemia, but study results are contradictory.104 Hypovitaminosis E leads to oedema in the enamel organ of rats.692 In hypervitaminosis E, the calcium and phosphate levels in teeth are increased, but the consequences of this (in humans) are not known.
Other Vitamins
Hypovitaminosis K could in principle underlie enamel defects as it lowers the calcium levels in plasma. Effects of a deficiency state of other vitamins are not described in the literature.
Systemic Disorders Due to Decreased Uptake of Dietary Substances
Malabsorption or the inability to respond to a regular supply of a nutritional component may also cause enamel defects. Due to lack of data, diseases such as necrotising enterocolitis, a common medical complication of preterm infants, are not discussed here.796
Coeliac Disease
This inherited digestive disorder is an allergy involving a selective lack of T cell tolerance for gluten (proteins in grains), which leads to atrophy of the small-bowel villous mucosa. Coeliac disease is the most common cause of malabsorption in the industrialised countries.8 Patients do not tolerate gluten in wheat, oat, rye and barley, but they can tolerate other proteins. Glutamine is enzymatically (transglutaminase) transformed into glutamine acid, to which gliadin is linked. The complex consisting of the two proteins sensitises and provokes the allergic reaction.908
In patients with coeliac disease consuming gluten, the absorption of other dietary substances is deranged. Clinical features include diarrhoea, weight loss, anaemia (iron deficiency), spontaneous fractures (due to deficient vitamin D and calcium), dermatitis herpetiformis, sometimes in only one location (elbow), diabetes mellitus and delayed puberty. Today, the clinical picture of the disease often is atypical.9 11
In the Netherlands, the prevalence of coeliac disease is approximately 2: 100 000, varying by region and city due to diagnostic reasons and dietary differences. In the UK, the prevalence is twice as high.429 One paper estimates 1% of the population may be affected.584
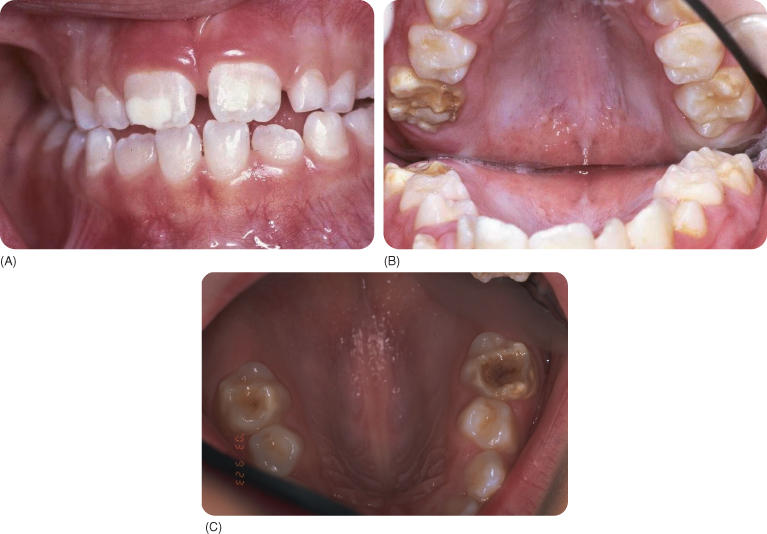
The teeth (70–75%, mainly incisors and molars, in any quadrant) of most patients (80–95%) show defects that are chronologically distributed (Figure 3.9). The tooth defects may be the only sign of the disease; a gluten-free diet reduces the risk of development of enamel defects. Seemingly healthy family members may also show dental lesions, which is an indication to screen them for the disease. In coeliac disease, it is the immunological response, and not the hypocalcaemia, that causes the defects in the enamel.8 9 11 549 559
Figure 3.9 (A–D) The teeth in coeliac disease, classified on basis of discoloration and hypoplastic enamel as I–IV.9
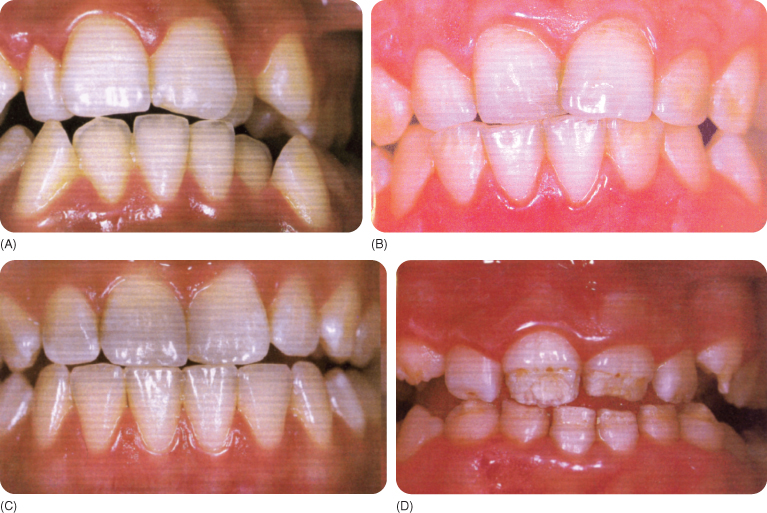
The patients typically have leucocyte antigens (DR3 and DQ2),8 while DR5,7 seems to protect against enamel defects.539 The coeliac-type enamel defects are classified into:
The teeth of both dentitions are severely hypomineralised with shortened, irregularly distributed prisms and decreased interprismatic substance.1036
The diagnosis is based on biopsy of the mucous membrane of the small intestine, and is confirmed with a second biopsy 1 year after start of treatment and a gluten provocation test to exclude patients who have a temporary intolerance. The provocation test is not performed before the age of 6 years in patients presenting with tooth defects suspected of having coeliac disease.721 An immunochromatographic assay for IgA and IgG antibodies to transglutaminase may be used instead of the biopsy.833
Late detection of coeliac disease in adults, owing to an atypical course of the disease, may have serious consequences: some 10% of unexplained osteoporosis cases are ascribed to the disease, and about 5% develop T cell lymphoma.584
Hypophosphataemic Vitamin D-Resistant Rickets (Hypophosphataemia)
Vitamin D-resistant rickets (“refractory rickets”) resembles hypovitaminosis D, notwithstanding a normal vitamin D status. An autosomal dominant292 316 (“hypophosphataemic bone disease”)788 and a X-linked form381 793 (“familial hypophosphataemia”)4 exist. Additionally, an autosomal recessive form has been reported.282 291 292 293
Hypovitaminosis D is rapidly remedied with small amounts (up to 50 µg/day) of the vitamin, but vitamin D-resistant rickets requires large doses, hence its name.108 215 Several forms of the disease exist.98 Adults show active and inactive hypophosphataemia with and without post-rachitic deformities. Another form is the vitamin D-resistant hypophosphataemia, which requires treatment with large vitamin D doses (but there is risk of hypervitaminosis).
In spite of treatment at an early age, some rachitic skeletal effects, such as a mild bowing of the legs, invariably remain.174 The first symptom may be a delay in walking caused by deformed legs (bowing) at approximately 2 years of age,681 but osteomalacia is often so mild that the dental manifestations are the first indication of presence of the disease.793
Dental Manifestations
Some patients show few or mild effects but others develop severe anomalies.806
- Alveolus development and calcification is poor in untreated patients and leads to loss of the lamina dura and periodontal ligament.174 Chronic periodontal disease may be present.1025
- The enamel may be normal,4 but is mostly hypoplastic, thin and yellow,924 with clefts.49 300 328 355 365 561 670 981 The enamel may be free of caries even when microorganisms have penetrated the dentine.390 Enamel hypoplasia and diffuse hypocalcification seem more frequent in the autosomal than in the X-linked form.788 At the time of its maturation, the enamel of vitamin D-resistant pigs was found to contain large amounts of non-degraded amelogenins.520 Various degrees of enamel fractures and wear have been reported.174 In the hypovitaminosis D form of rickets the incisal and occlusal enamel is hypoplastic more frequently as well as to a greater extent than in hypophosphataemia.108
- The amelodentinal junction is histologically quite normal.
- The dentine is primarily affected. The mantle dentine is normal,390 the deeper dentine is hypoplastic, thin, globular and poorly mineralised.154 174 799 806 981 The more severe the disorder, the greater is the amount of globular dentine,4 126 807 924 which is graded as (in deciduous teeth); I (<50% globular); II (>50% globular); and III (almost 100% globular).807 The predentine is wide and there is extensive reparative dentine formation.39 Although a diet rich in calcium and phosphate lead to increase in the serum phosphate level in vitamin D-resistant mice, the amount of interglobular dentine did not decrease,3 387 which is indicative of genetic influences.387 The dentinal calcium : phosphate ratio in rickets is increased.4 The teeth contain more sodium and magnesium than normal, and the interglobular spaces contain more zinc than other parts of the tooth, but the significance of these findings remains unexplained.387
- Tubule-like extensions (or microcracks) in the dentine around the pulpal extensions connect the pulp horns of the large pulp chamber4 520 788 with the amelodentinal junction and enamel clefts, with fissures linking the enamel subsurface to the pulp horns.540 The pulp may become necrotic without any sign of caries,4 126 681 924 or even when any decay has been adequately treated.154 In severe cases, periapical abscesses are common.381 793 807 Sinus tracts may be the first sign of the disorder, especially in the deciduous dentition.806 799 1019
- The cementum has also been found to be abnormal: there is less cellular cementum and irregular calcification.174 Root completion and eruption are delayed.316 365 520 561
Taurodontism has been observed in more severely affected males.328 In hypovitaminosis D, the prenatal dentine is abnormal900 and interglobular dentine develops postnatally.355 561 Angle’s Class III malocclusion is frequently seen in the X-linked form.788
Epidemiology
Vitamin D-resistant rickets (prevalence 1 : 20 000) affects more girls than boys,108 788 although the reverse has also been documented.583 Boys show more severe anomalies.300 799
A substantial number of (untreated) patients have multiple periapical abscesses in the deciduous and/or permanent dentition, even in caries-free and clinically normal teeth,48 174 328 583 710 1037 especially in the deciduous dentition.583 The medication dose is an unreliable predictor of abscess occurrence.583 The younger the patient when the first abscesses occur, the more severe the dental manifestations of the disease.799 Wear of the enamel and the defective dentine accounts for the microbial invasion of the pulps of deciduous teeth, but the reason why the pulps of permanent teeth become necrotic remains unexplained in a number of cases. In the absence significant wear, enamel infractions may provide a route of entry for the microorganisms.328
Aetiology
The tubular reabsorption of inorganic phosphate in the kidneys and the gastrointestinal uptake of calcium is below normal, and this affects women more than men.4 300 355 456 550 In X-linked rickets, the availability of calcium is the main problem, although some researchers have implicated phosphate. The calcium level is, however, normal.788 The disorder is attributed to the inability to form hormone D3 due to a deficiency in the enzyme 25(OH)-D-1-hydroxylase.291 292 293 1025 Hypocalcaemia due to insufficient D3 hormone leads to hyperparathyroidism, which partly corrects the calcium plasma level, but impairs the reabsorption of phosphates in the renal tubules, causing hypophosphataemia.154 292 293 The mineralisation of bone is thus defective.550
In autosomal dominant rickets, the amount of D3 hormone is sufficient; but the hormone D3 receptors in all kinds of cell are abnormal.456 In the X-linked and autosomal dominant disorder the serum values for phosphate are similar.788 In the autosomal form, the alkaline phosphatase and D3 hormone levels are persistently high.456 The deficiency in phosphates caused by other diseases and syndromes may be expected to result in dental anomalies, but not much is known in this regard.998 For instance, hypophosphataemia caused by an inherited excessive renal excretion of phosphates increases the production of D3 hormone.889 Effects on the teeth are not reported, but might exist via a vitamin D-dependent calcium-binding protein in the ameloblasts.885
Prevention and Therapy
The d/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


